Introduced in Canada over two decades ago, Patient Support Programs (PSP) have evolved from providing drug bridging and financial support to a more comprehensive portfolio of services including, but not limited to: providing infusions, injection support, life coaching, and access to allied health professionals. The role of PSPs has become increasingly important in enhancing the care of patients as they begin their treatment journey. At the same time, there has been a growing need for manufacturers to generate real world evidence (RWE). Stakeholders, including payers, healthcare practitioners (HCPs) and even patients, want to understand the real-world effectiveness and safety of therapies, in order to more clearly define a drug’s value.
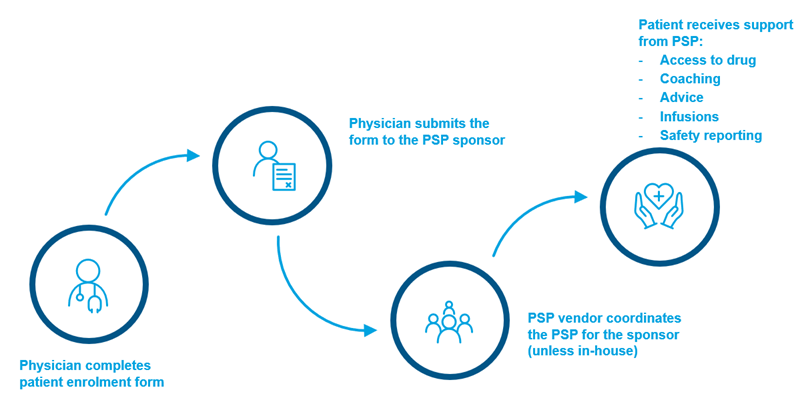
Figure 1: Facilitating Patient Enrolment in a PSP
Studying PSP data is an efficient and cost-effective means to generate Real World Evidence
PSPs collect a wealth of patient-level data to facilitate reimbursement, including information such as patient characteristics, drug utilization and certain clinical outcomes. Since these data are already captured by a manufacturer’s PSP, there is an opportunity to tap into this pre-existing wealth of information. With appropriate consent and scientific rigour, robust RWE insights can be generated in a timely and cost-effective manner.
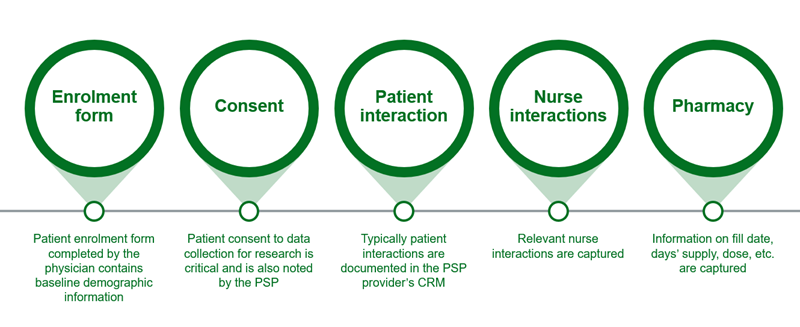
Figure 2: Information collected in a PSP
Drawing accurate insights from PSP data requires understanding the specific data fields captured, the purpose of data collection, and the data gaps that exist. Without this context, there is risk that the data can be misinterpreted or false conclusions drawn, thereby decreasing the scientific validity of the results.
IQVIA Real World Solutions has over 5 years of experience conducting RWE studies from PSPs
The IQVIA Real World Solutions (RWS) team conducted our first PSP study in 2015, which resulted in three published manuscripts [1-3], and several conference presentations [4-9] from 2016 to 2018. Since the inception of our first PSP study, we have continued to refine and streamline our processes to become a Canadian leader in generating real world evidence from PSPs, ranging from rare disease, to oncology to more prevalent conditions such as respiratory, neurology, and infectious diseases. By leveraging our expertise in data analytics and integrating our understanding of trends in the pharmaceutical industry, we have successfully partnered with manufacturers to maximize the value of their PSPs. This has included recommendations on ways to enhance data collection by incorporating tools that capture patient reported outcomes (PROs), to leveraging other data sets to plug in any data gaps.
IQVIA has leveraged our position as a leading Canadian RWE organization to develop collaborations with external data sources including some provincial administrative databases that capture publicly funded services, which can be leveraged to help manufacturers optimize the insights they can obtain from their PSPs. This is particularly useful to help manufacturers answer questions such as: does the therapy help reduce hospitalizations and what is the impact of improved adherence as a result of the PSP on outpatient costs? Generating this evidence can help develop compelling arguments for both internal and external stakeholders about the value of the PSP and therapy on overall health outcomes.
It is important to note that robust data analysis from a PSP cannot be done without considering privacy and data confidentiality issues. Accordingly, IQVIA can provide input on how to ensure that the data collection does not compromise patient privacy or patient care. We securely transfer de-identified patient-level data from the PSP database into a secure study database thereby separating the data required for RWE generation from the patient identifiable information used for the program. This maintains patient confidentiality while enabling data analysis.
As depicted in the figure below, we have built a process that takes into account the various components of a PSP study: security of data, integration of several data sources if needed, and a methodological approach to ensure we are able to generate insights that can be looped back into the PSP and used by multiple internal stakeholders.
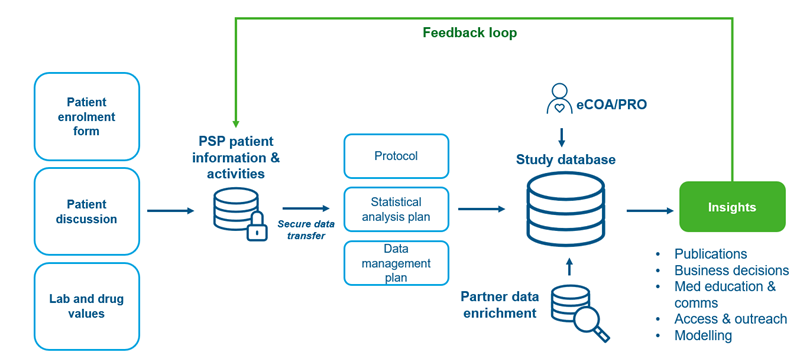
Figure 3: Leveraging a PSP to Generate RWE
eCOA: electronic clinical outcome assessment (provided by the HCP)
PRO: patient reported outcomes (provided by the patient)
In conclusion, PSPs represent a wealth of data, collected and potentially available for analysis. Being able to draw accurate, relevant and timely insights from PSPs can demonstrate improvements in patient care and help communications with stakeholders. When done with sufficient scientific rigour, these can also be published in peer-reviewed literature. Our team of epidemiologists, statisticians, medical and market access experts have established themselves as trusted partners with pharmaceutical manufacturers, employing innovative approaches when analyzing PSP data to generate compelling and informative RWE insights.
If you would like to find out more about how we can help you conduct PSP studies, please contact Calum Neish at calum.neish@iqvia.com
References
- Marshall, J., et al., Canada's Study of Adherence Outcomes in Patients Receiving Adalimumab: 3-year Results From the COMPANION Study. (1879-114X (Electronic)).
- Narula, N., et al., Impact of Adalimumab Patient Support Program's Care Coach Calls on Clinical Outcomes in Patients with Crohn's Disease in Canada: An Observational Retrospective Cohort Study. (2515-2092 (Electronic)).
- Bessette, L., et al., Impact of the Adalimumab Patient Support Program on Clinical Outcomes in Ankylosing Spondylitis: Results from the COMPANION Study. Rheumatology and therapy, 2018. 5(1): p. 75-85.
- Marshall, J.K., et al., Impact of the Adalimumab Patient Support Program's Care Coach Calls on Persistence and Adherence in Canada: An Observational Retrospective Cohort Study. Clinical Therapeutics, 2018. 40(3): p. 415-429.e6.
- Bessette, L., et al., Canadian Study of Adherence Outcomes in HUMIRA® (Adalimumab) Patients – Three-Year Results from the COMPANION Study in Rheumatology Patients, in Canadian Rheumatology Association. 2017: Ottawa, Canada.
- Marshall, J., et al., Canadian Study of Adherence Outcomes in HUMIRA® (Adalimumab) Patients: Three-Year Results from the COMPANION Study in Gastroenterology Patients, in Canadian Disgestive Diseases Week Annual Scientific Conference. 2017: Banff, Canada.
- Gerega, S., et al., Canadian Study of Outcomes in Adalimumab (HUMIRA) Patients with Support for Adherence - Results from the Companion Study, in American College of Rheumatology. 2016: Washington DC, USA.
- Shear N; Bessette L, M.J., Lebovic G; Gerega S; Millson B, Gaetano T; Gazel S; Latour M; Laliberte M, Canadian Study of Outcomes in HUMIRA® (Adalimumab) Patients with Support for Adherence - Dermatology Results from the COMPANION Study, in Dermatology Update. 2016: Montreal, Canada.
- Latour, M., et al., Canadian Study of Outcomes in HUMIRA® (Adalimumab) Patients with Support for Adherence - Results from the COMPANION Study, in American College of Gastroenterology 2016: Las Vegas, USA.
- Marshall, J., et al., Impact of adalimumab’s patient support program on clinical outcomes in inflammatory bowel diseases: Results from the COMPANION study, in European Crohn's and Colitis Organization. 2017: Barcelona, Spain.







