











Digital engagement with HCPs is slowly and surely becoming the core of your outreach and marketing communications. You recognize the need for a digital first strategy but pervasive myths about the current landscape may prevent you from leveraging these channels to their full potential. Here are three common myths and how to prepare for a digital first future.

HCPs have been forced to adapt and re-balance their priorities as the patient backlog increases due to lockdowns and restrictive measures. This has, in turn, led to downward pressure on their time and how they use it. While seeing patients continues to be the top priority for physicians, the way that they interact with patients has changed from face-to-face interactions to remote channels (namely telemedicine).
Physicians have adopted telemedicine to connect with patients
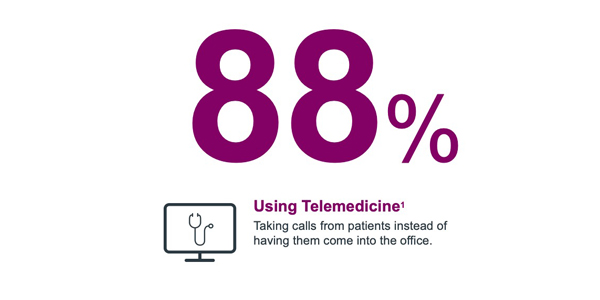
This transition has significantly and negatively impacted physicians’ time to see and interact with pharma reps in person. This has led HCPs to explore remote engagement channels -- almost all of them digital. In fact, now, more than 50% of physicians would like to engage digitally and remotely given the pressures they face.
Virtual engagement preference change after COVID-19 restrictions
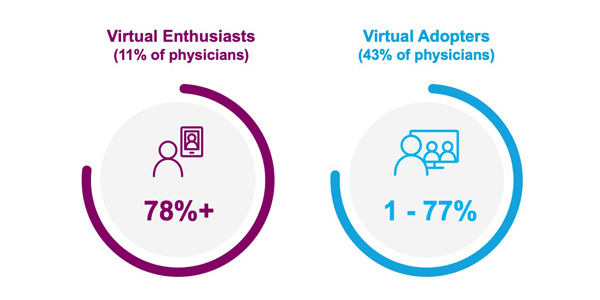
Fifty-four percent of physicians prefer a future with more virtual engagement.
While it can be tempting to brush this off as a restrictions-related reaction by HCPs, our research on future engagement preferences show otherwise.
Physicians' continued use of virtual (telehealth/remote consultation) is primarily driven by increased experience and familiarity with remote channels acquired during COVID lockdown.
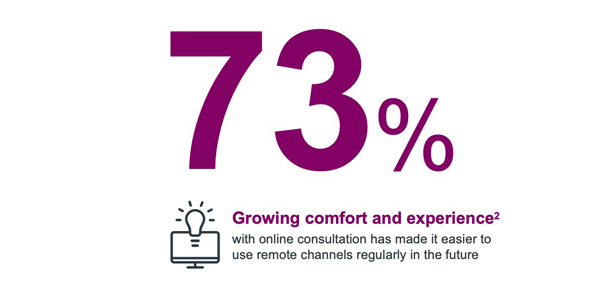
These seismic shifts are here to stay, and will no doubt influence the future of engagements.
Pharma companies need to not only prepare for, but also find a way to thrive in, the next normal. It all starts with identifying and predicting physician segments (who to reach) of your high-value HCPs and start establishing a ‘digital’ relationship by obtaining their permission to contact them through electronic means.

While your field teams may have consent to engage HCPs directly, it becomes difficult to apply those consents for enterprise communication and outreach. Let me explain.
In Canada, communicating to HCPs using electronic messages is slightly more complex given Canada’s Anti Spam Legislation (CASL), the federal law which deals with spam and other electronic threats.
At its core, CASL prohibits companies from sending commercial electronic messages without consent, including email, social media and text messages. Consent can exist in two forms:
- Express Consent
- The recipient must take a proactive action to indicate their express consent
- This consent is not time-limited
- Implicit Consent
- Includes having an existing business relationship (EBR) based on a previous commercial transaction with the recipient
- EBR exists if a transaction occurred within 2 years of the email date OR inquiry was made within 6 months of the email date (grace period expired July 2017)
Source: From Canada’s Anti-Spam Legislation (CASL) Guidance on Implied Consent | CRTC
So, unless your sales force was clearly mandated to capture express consent with proof of that consent for every HCP, they have likely gathered implicit consents given their EBR, as defined above. It also becomes difficult to track and maintain these consents if reps leave your organization or when your target universe is in the hundreds or thousands. Consequently, you are better served when express consents are collected and maintained, independent of your field teams.
We strongly encourage pharma companies to obtain express consent for three reasons – assets, audits and access:
- Asset development: In capturing express consents, your company develops its digital contact list as an asset. This helps your organization own and manage messaging, timing, frequency and recency from an enterprise level rather than at the individual rep level, with all the variability that can result.
- Improved auditing: In the case of an audit, having proofs of consent is highly preferred. Implicit consent is not ideal., Defining an existing business relationship with a specific email address is difficult. Most pharma companies have existing mechanisms to manage express opt-outs, however, many do not have the capabilities to track expiry of implicit consent. This becomes an administrative burden that is best avoided.
- Broader access: With these consents, other digital touch points can be leveraged (chatbots, social media, text etc.) for a complete digital multi-channel outreach strategy.

Consent capture, if executed as a standalone project, can come across as narrow in scope and not worth the investment. You can consider taking a different approach that leverages existing marketing materials and integrates consent capture as part of your ongoing marketing plan and communications.
We recommend a model of “Start Anywhere, Go Everywhere” to obtain digital permissions. It doesn’t matter when you want to start as long as you have a message that acts as a strong hook to engage. Adapting your existing (or planned) marketing messages, such as new product launches, webinars, CMEs, formulary changes or anything that will be of value to the HCP, is critical. It must be compelling enough for the HCPs to say they want to stay in touch with your company.
Furthermore, an HCP may not provide consent right away but will do so over time and with relevant messaging. For example, an outreach just before or during flu season (vs summer) to physicians focused on respiratory illnesses, has a significantly better chance of obtaining consent. A program with multiple waves of outreach at different times of the year, based on a strong value proposition for the HCP, yields far better results.
A programmatic approach that leverages your existing marketing calendars, outreach collateral, and creative assets is key. And why not? You have already invested and planned for the next marketing cycle, so use it to your advantage.
Additionally, if you already have classified physician segments (Tier 1,2,3,4) this is the best place to start making your data work a bit harder. Start by securing permissions from the targets that are most valuable to you. Now, you are armed with knowing where you should concentrate your multi-channel outreach (MCO) and assign the most effective channel mix (phone, fax, direct mail) to each segment.
Ensuring success requires testing each element – frequency, layouts, messaging and scripts – before executing the actual campaign. Capturing the metrics on channel performance, HCP preferences, and the proofs of consents provided back gives you the necessary campaign intelligence of what has worked and what hasn’t. This critical information can be used to design and adapt the next set of campaigns.
Taking a programmatic approach (see below) versus dealing with this as a one-off project helps you be consistent with your brand or corporate messaging, ensures your current investments in data and creative development goes further, and helps build your digital first strategy.
A Comprehensive MCO Solution leverages investments in e-consent blitz through ongoing collection and communications.
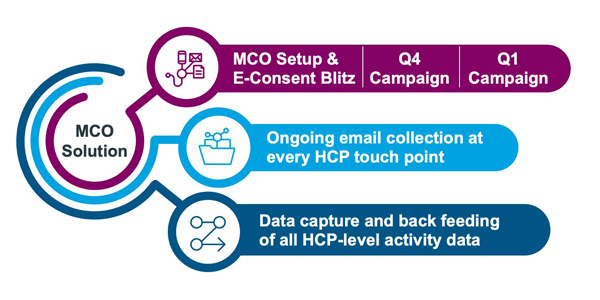
Email consent gathering is an ongoing process. Each touchpoint, regardless of the channel, is an opportunity to continue to grow your database of CASL-compliant email addresses. Once the initial eConsent ‘blitz’ is complete, IQVIA can orchestrate your next brand-specific campaigns by adding non-email channels where required to provide complete coverage of your market.
This is an exciting time for pharma marketers who MUST go digital. No matter where you are in the consent or outreach process, IQVIA can help you establish a strategy and see it through to execution so you can engage better with your audience.
Over time, these digital connections can be quickly complemented by co-ordinating face-to-face high-value touch points by customer-facing teams (MSLs, KAMs, Sales Reps, etc.) for a hyper-connected one-to-one experience. This helps pharma companies break down internal silos and get to a hybrid “digital first” commercial go-to-market model that puts the HCPs at the core of all interactions. Pharma companies can now start future proofing their outreach communications and lay the foundations of orchestration between sales and marketing.
Our next blog will discuss obtaining digital permissions and how to create digital journeys for your HCPs for a hyper connected experience. Stay tuned.
To learn how you can strengthen your digital connections by getting explicit consent working for your organization, please contact CanadaInfo@iqvia.com.

