Meet the challenge of changing stakeholder demands and increasing cost constraints with IQVIA's integrated technology services and analytics-driven offerings.






















- Blogs
- IQVIA Regulatory Artwork Management Services
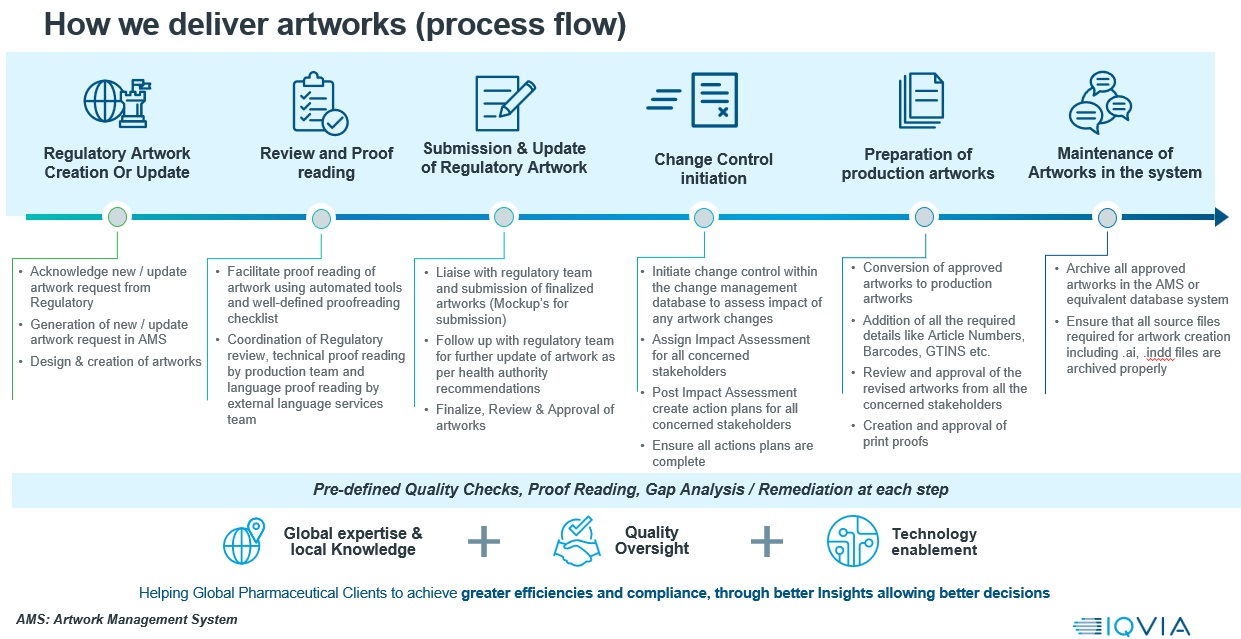
IQVIA Regulatory Artwork Services offers comprehensive end-to-end artwork solutions for the entire life sciences industry, encompassing Pharmaceuticals, Medical Devices, Cosmetics, and Consumer Healthcare.
IQVIA provides designs for a broad range of product categories, including both digital and print media, from primary & secondary packaging designs to promotional materials. IQVIA also provides fully managed regulatory artwork services that cover the entire spectrum of artwork coordination, regulatory review, creation, and proofreading.
Our artwork solutions combine cutting-edge technology, seamless digital connectivity, and adherence to validated Good Manufacturing Practices (GMP) workflows.
IQVIA Regulatory Artwork Services Overview:
- Team Size: Comprising 30+ artwork specialists, the IQVIA regulatory artwork team is fully dedicated to the artwork hub. Our expertise spans designing, creation, proofreading, and regulatory consulting for artworks.
- Sponsor Engagements: With a track record of supporting 20+ sponsors, the labeling and artwork team consistently delivers excellence.
- Artwork Production: Annual production of 5,000+ artworks, ensuring high-quality visual materials across various purposes.
- Market Experience: The team provides robust support to 110 markets for regulatory artwork design and creation.
- Proofreading: Experience of proofreading over 20,000 pieces, the artwork team maintains accuracy and excellence, consistently meeting 100% quality KPIs.
- 24/7 Coverage: Our commitment extends around the clock, providing continuous support and ensuring timely deliveries.
Why a Robust Pharmaceutical Artwork Partner Is Essential
In the complex and highly regulated world of pharmaceuticals, the role of artwork cannot be underestimated. The right artwork partner can make a significant difference in these key areas:
Quality Enhancement for Commercial Packages:
- First Impressions Matter: Commercial packages are often the first interaction patients have with a medication. High-quality artwork ensures that the packaging conveys professionalism, trust, and reliability.
- Accurate Information: Precise labeling and clear instructions prevent confusion, reduce errors, and enhance patient safety. A reliable artwork partner ensures that every detail—from dosage to warnings—is flawlessly presented.
Navigating Regulatory Changes:
- Dynamic Landscape: Regulatory artwork requirements evolve constantly. A skilled artwork partner stays abreast of changes, ensuring compliance with guidelines from agencies like the FDA, EMA, and others.
- Risk Mitigation: Non-compliance can lead to costly delays, recalls, and even legal repercussions. A proactive partner anticipates regulatory shifts and adapts swiftly.
Minimizing Recalls and Shortages:
- Costly Consequences: Product recalls due to artwork errors can be financially devastating. A diligent partner minimizes the risk of recalls by maintaining accuracy and consistency.
- Supply Chain Stability: Artwork delays can disrupt supply chains, leading to shortages. A reliable partner ensures timely approvals, reducing the chances of stockouts.
Cost Efficiency in Artwork Management:
- Streamlined Processes: An efficient regulatory artwork partner optimizes workflows, reduces revision cycles, and minimizes waste. This translates to cost savings and improved efficiency.
- Right First Time: Accurate artwork reduces rework, saving both time and resources. A strategic partner focuses on getting it right from the outset.
Speed to Market:
- Critical Timelines: Swift market entry is essential. A responsive partner expedites regulatory approvals, allowing products to reach patients faster.
- Competitive Edge: Being first to market can significantly impact revenue. An agile partner accelerates the launch process.
IQVIA Regulatory Artwork Services: Why choose the IQVIA Team?
In the complex world of regulatory artwork services, the choice of a partner can significantly impact your business outcomes. A partner that brings together global expertise, technical excellence, cutting-edge technology, and a versatile team can be a game-changer. IQVIA Regulatory Artwork Services is one such partner that stands out in this competitive landscape. Choosing IQVIA can be a strategic advantage for your organization, including:
Global Expertise and Coverage
- Worldwide Reach: IQVIA operates across 120 countries, providing a truly global perspective.
Zero Refusal to File
- Technical Excellence: IQVIA’s meticulous approach eliminates label technical errors, resulting in zero refusals to file from regulatory agencies.
In-house Technology
- Pixel Proof and Text Proof: Our cutting-edge technology ensures pixel-perfect and error-free artwork.
- Label Compare for Compliance: End to end comparison of all label types.
- Labeling Intelligence Hub: Intelligence driven labeling decisions.
- AI driven Orchestrated Workflows: Integrated and intelligence driven workflows.
Technology Agnostic Team
- Versatility: Our team is adept at multiple standalone tools, adapting to your specific requirements.
- Augmented: We blend technology with human expertise for optimal results.
Client Benefits
- Integrated Process with Zero Errors: Our end-to-end approach minimizes errors, ensuring seamless artwork management.
- Rework Minimization with Harmonized Process: Efficient workflows reduce rework, saving time and resources.
- Cost Savings: IQVIA’s strategic solutions optimize costs without compromising quality.
Case Study: Streamlining Artwork Management for a Global Client
Client Background
The client is a global company with products sold in over 60 countries and more than 1000 SKUs. The initial artwork creation and artwork change management are being managed by IQVIA.
Challenges
The client faced several challenges in their artwork management process:
Manual Artwork Process: The process was manual with long review workflow cycles.
Communication: The email-based communication resulted in a significant reduction in efficiency and delayed artwork process management.
Consistency: Maintaining consistency across the products globally with a large number of artworks was a major challenge.
Process Deviations: There were issues with process deviations and adherence.
Solutions
- An end-to-end artwork management system was put in place.
- The client’s SOPs were updated as per the current market standards.
- Quality gateways were set up to reduce deviations/errors.
- End-to-end support of artwork creation, coordination, proofreading, serialization, and repository maintenance was provided.
- A client-specific article number system was implemented to track GTIN numbers, serialization requirements, and site-specific article numbers.
- SLAs and KPIs were set up to ensure the right first-time approach.
Results
The implementation of these solutions led to significant improvements:
- The overall process efficiency increased by 20% with zero quality issues for the last 2 years.
- Manual activities were reduced, and update, review, and finalization timelines were reduced from five weeks to three weeks.
- Automation reduced manual efforts by eliminating line-to-line checking of text components in the quality check process with automated comparison.
Quality and functional metrics were monitored as per the agreed SLA, which helped to enhance the overall process and quality efficiency.
Related solutions
Reimagine regulatory service delivery.
Automate and standardize your regulatory management, from correspondence and commitments to registration and tracking.





