Connect chemical buyers and sellers or alternative sources with ease in an ever-increasing competitive environment. IQVIA Chemical Intelligence is the solution to give you access to the most current and accurate information when and where it’s needed.






















- Blogs
- Safeguarding Human Health and Environment
Introduction – REACH Objective
REACH stands for Registration, Evaluation, Authorisation, and Restriction of Chemicals. It is an important regulation implemented by the European Union (EU) with the aim of safeguarding human health and the environment from the potential risks associated with chemicals. It intends to bolster the competitiveness of the EU chemicals industry through product safety which helps to build consumer confidence and trust. It facilitates market access by providing evidence of compliance, promoting safer and innovative practices. It also encourages collaboration and transparency between suppliers at different levels within a supply chain by prompting manufacturers to supervise and assess the risks posed by raw materials used for manufacturing their product. Also, through this regulation, the EU aims to foster innovation and sustainable practices within the industry, encouraging the development of safer chemical products and processes.[1]
Fundamentals of REACH
1. Registration
Under REACH, companies are responsible for proving the safety of the substances they produce to the European Chemicals Agency (ECHA) and communicate the necessary risk management measures to users. Companies need to maintain a registration dossier to submit to ECHA, which contains hazard information, and if applicable, an evaluation of the risks posed by the substance and recommended control measures.
The principle of "one substance, one registration" is followed for registration. This means that manufacturers and importers of the same substance may need to collaborate with each other and submit their registrations jointly. A registration fee is typically charged for the submission of a substance registration.
Manufacturers, importers, downstream users, and EU-based representatives of non-EU manufacturers that manufacture or distribute products in EU should mandatorily hold REACH certification.[1]
Identification of Substance
Substance identity is commonly described by the chemical name of the substance, its EC number (identifier assigned to commercially available chemicals in EU) and chemical composition determined through chemical analysis. Substance identification facilitates efficient and correct preparation of joint REACH registrations, ensures appropriate test data for the registered substance, and enables a robust assessment of its hazards and risks. Accurately identifying a substance helps prevent unnecessary animal testing, efficient utilisation of test data and checking regulatory status of the substance. [1]
2. Evaluation
Once submitted, ECHA evaluates individual registrations for compliance, while EU member states assess selected substances to address initial concerns regarding their impact on human health and environment and determines if the risks associated can be effectively managed. The evaluation process involves reviewing testing proposals, checking compliance of registration dossiers, and conducting substance evaluations. Following the evaluation, registrants may be requested to provide additional information about the substance. [1]
3. Authorisation
The aim of the authorisation process is to ensure effective risk management for substances identified as substances of very high concern (SVHCs) by ECHA or other EU member states and promote their substitution with safer alternatives, considering technical and economic considerations. The restricted substances or SVHCs include those classified as carcinogenic, mutagenic, or toxic for reproduction (CMR), persistent, bio accumulative, and toxic (PBT) and substances with equivalent concerns.
If a substance is identified as a SVHC, it is included in the Candidate List for restricted substances. Listed SVHCs impose obligations on suppliers, including providing safety data sheets, communicating safe use information, responding to consumer inquiries, and notifying ECHA of SVHC presence in products above a concentration of 0.1%.[1]
4. Restriction
Restrictions aim to limit or prohibit the manufacturing, marketing, or use of substances where risks cannot be adequately controlled. ECHA can choose to restrict certain substances or ask for prior authorization for specific uses of substances. [1] Lead is an example of a chemical restricted by REACH. It is subject to specific concentration limits and labeling requirements in products like toys, jewelry, and certain electrical/electronic equipment. Technical measures, such as protective equipment and safe handling procedures, are implemented to minimise Lead exposure and ensure compliance with REACH regulations for the safe use of lead-containing products. [2]
Restrictions are applicable to substances used individually, in mixtures, or present in products. Ultimately, the aim is to gradually replace the most hazardous substances with less dangerous alternatives.
Exemptions from restrictions include on-site isolated intermediates, substances used in scientific research and development, and substances posing risks solely in relation to cosmetics use. [1]
Once the required conditions are met, ECHA issues the REACH certificate which will have a REACH registration number. [3]
Figure 1. REACH Approval by ECHA – Process Flow [4]
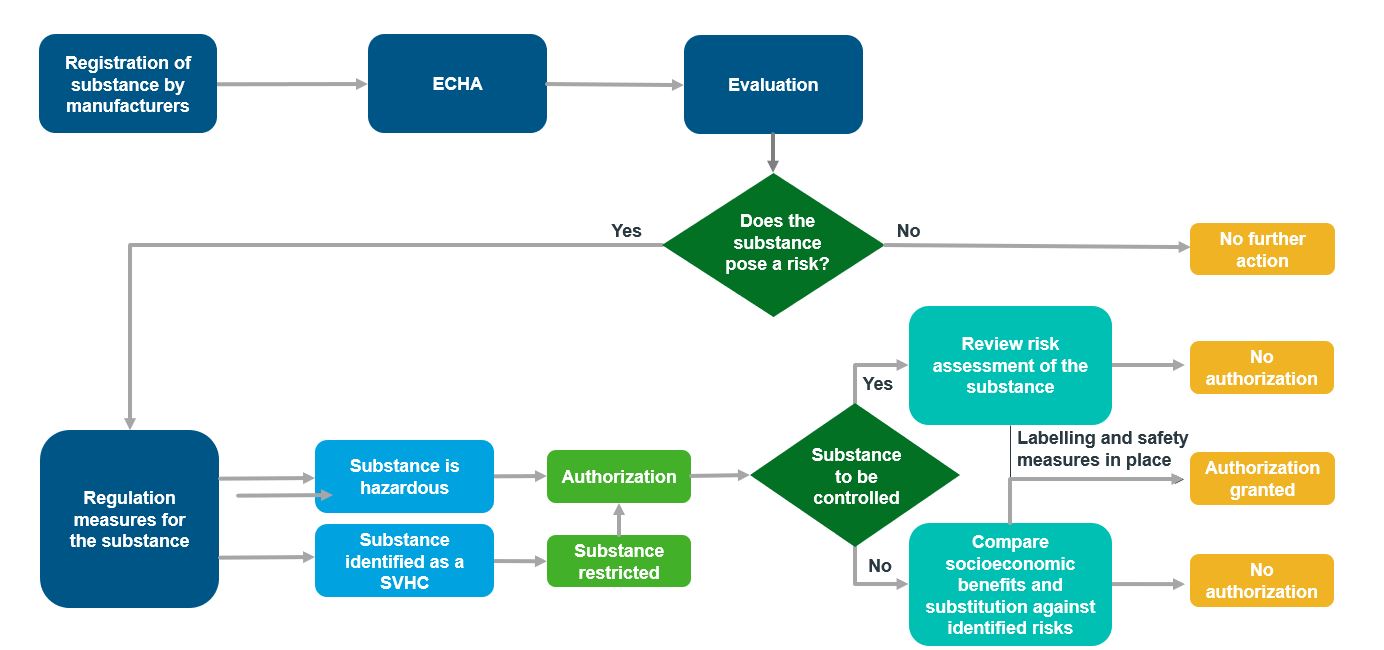
Source : https://www.researchgate.net/figure/Flow-Chart-Summarizing-REACH-Registration-Evaluation-and-Authorization-of_fig1_7952389
To determine the effects of chemicals on human health and environment, companies must test their chemicals for safety. This entails conducting appropriate tests or trials on animals. It is worth noting that ECHA mandates companies to use alternative testing methods wherever possible and use animal testing as a last resort. ECHA is actively involved in determining the necessity of testing and provides regulatory expertise. It also invests in platforms for chemical data, such as International Uniform Chemical Information Database (IUCLID) and the Quantitative Structure–Activity Relationships (QSAR) Toolbox to reduce the need for animal testing.[1] IUCLID is a central repository that allows companies to store and submit data on chemical substances, facilitating information sharing and collaboration. [5] The QSAR Toolbox is a software tool that utilises computational methods to predict the properties and behavior of chemicals, reducing the reliance on animal testing. [6]
The practice of joint registration for the same substance by different companies is one such example that helps avoid duplicate testing. Other approaches supported by ECHA to reduce animal testing include use of computer modeling tools and collaborations to exchange data and best practices globally. [1]
Brexit & UK REACH
Brexit refers to the United Kingdom's exit from the European Union on 31st January 2020. Brexit has had significant implications for the application of the REACH regulation in the United Kingdom (UK). [7] European Union / European Economic Area companies with supply chains involving the UK are affected by Brexit. The EU chemicals legislation, including REACH, no longer applies to their operations in the UK if they only place chemical products on the UK market. [1] The EU REACH Regulation was adopted into UK law as UK REACH through the European Union (Withdrawal) Act 2018. UK REACH replicates the key principles of EU REACH with necessary modifications for domestic implementation. Both UK REACH and EU REACH operate independently, so compliance with both regulations is necessary wherever applicable. UK REACH governs chemicals placed on the market in Great Britain, while EU REACH continues to apply in Northern Ireland under the Northern Ireland Protocol. [8]
Conclusion
The main objective of REACH is to safeguard human health and the environment by holding businesses responsible for demonstrating the safety of chemicals. It promotes the use of safer alternatives, encourages information exchange, and enables the control of substances of high concern. The REACH certificate provides information to consumers about the substances and chemicals used in products, allowing them to make informed choices based on the safety and potential harm of these substances. REACH certification is mandatory if a company is manufacturing in the EU/exporting chemicals to the EU in quantities exceeding one ton per year. Failure to acquire a REACH certificate could result in a company getting banned from the EU for that product.
Considering the significance of REACH in the chemical supply chain, IQVIA Chemical Intelligence has recently integrated this data into the Chemical Intelligence database. REACH data can be accessed in the Directory of Producers module which serves as a comprehensive resource that connects producers and buyers of raw materials, specialty chemicals, and bulk pharmaceuticals in more than 100 countries. Using this module, customers can actively reach out to companies that manufacture REACH registered products for their sourcing needs. The platform can also be leveraged by competitors aiming to enter the EU market by allowing them to identify active EU manufacturers, enabling a comprehensive assessment of the competition.
The Directory of Producers module offers a total of 489K+ products and 13K+ manufacturers which are associated with 21K+ certifications including REACH. This module helps customers identify producers and competitors and promotes connections within the global marketplace. It helps customers make informed choices and helps them to connect and negotiate with producers and other such entities fostering collaboration between stakeholders in the chemical industry.
IQVIA™ Chemical Intelligence is a leading information provider to purchasers, sellers, and researchers in the Pharmaceutical and Chemical industries worldwide. To find out more, please click here.
References
- echa.europa.eu/
- http://www.chemsafetypro.com/
- https://www.everythingrf.com/community/what-is-a-reach-certificate
- Lanphear, Bruce & Vorhees, Charles & Bellinger, David. (2005). ‘Protecting Children from Environmental Toxins’, PLoS medicine. 2. e61. 10.1371/journal.pmed.0020061.
- https://iuclid6.echa.europa.eu/
- https://qsartoolbox.org/
- https://www.government.nl/topics/brexit/
- https://www.hse.gov.uk/
About the Author
Chandana D, Global Data Associate, IQVIA Chemical Intelligence is a Master of Science graduate in Microbiology from Bengaluru Central University. She has been with IQVIA for a year and has experience in pharmaceutical data research and content development.





