Explore IQVIA by region
As a global community, IQVIA continuously invests and commits to advancing human health.
Americas
Europe
Asia & Oceania
Middle East & Africa

Regional Thought Leadership
SOLUTIONS
Research & Development
Real World Evidence
Commercialization
Safety & Regulatory Compliance
Technologies
LIFE SCIENCE SEGMENTS
Consumer Health
Emerging Biopharma
Generics
MedTech
Pharmaceutical Manufacturers
HEALTHCARE SEGMENTS
Information Partner Services
Financial Institutions
Global Health
Government
Patient Associations
Payers
Providers
Therapeutic and Specialty Areas
Therapeutic and Specialty Centers of Excellence offer scientific expertise, therapeutic insights and clinical trial experience to expedite new therapies for patients.
LEARN MORE
Therapeutic and Specialty Areas
Allergy & Respiratory
Biosimilars
Cardiovascular
Cell & Gene Therapy
Central Nervous System
Dermatology
Early Phase Clinical Development
Endocrinology
GI & Hepatology
Infectious Diseases & Vaccines
Nephrology
Obesity
Oncology & Hematology
Ophthalmology
Pediatrics
Rare Diseases
Reproductive Health
Rheumatology
Harness the power to transform clinical development
Reimagine clinical development by intelligently connecting data, technology, and analytics to optimize your trials. The result? Faster decision making and reduced risk so you can deliver life-changing therapies faster.
Research & Development Overview

Early Phase Clinical Trials
Track the impact of new drugs and establish proof of concept faster with innovative early clinical development strategies.
Phase IIb/III Trials
Advanced AI and technology help you navigate regulatory complexity, optimize enrollment strategies and drive patient engagement.
Patient & Site Centric Solutions
Support patients and trial sites with tools and services that improve recruitment, engagement, and study execution.
Functional Services
Agile, data-driven trial solutions that connect people, processes, and technology for faster, smarter R&D delivery.
Global Laboratories
Specialty and central lab solutions backed by scientific rigor and expertise in biomarkers and antibody drug discovery.
R&D Consulting
Optimize your R&D strategy, reduce risk and ensure compliance with data, analytics, AI-powered technology and expertise.
Real World Evidence. Real Confidence. Real Results.
Generate and disseminate evidence that answers crucial clinical, regulatory and commercial questions, enabling you to drive smarter decisions and meet your stakeholder needs with confidence.
REAL WORLD EVIDENCE OVERVIEW

Real World & Health Data Sets
Global real world data exploration at your fingertips enables critical insights across the product lifecycle.
Medical Affairs
Access end-to-end Medical Affairs solutions to plan, generate, and disseminate trustworthy medical evidence.
IQVIA Health Communications Group
Deliver communications that engages stakeholders, advances healthcare and improves lives.
Health Data Apps & AI
Streamline research process and gain access to real world data discovery with the largest agnostic health data catalog.
Health Data Transformation
Accelerate disparate health information into insight-rich, value-driving data transformation at scale.
Study Design
Identify the right study design to answer your research questions with unparalleled data, technology, AI and analytics.
Evidence Networks
Access global evidence networks to connect data, technology, and analytics for broader study options and collaboration.
Health Economics & Value
Generate the evidence you need to go from research to market, overcome regulatory hurdles, or support payer approval.
Regulatory and Safety
Explore options with innovative RWE study approaches to prove your product’s safety and effectiveness.
Natural Language Processing
Extract insights at scale from unstructured text with IQVIA’s advanced NLP platform—fast, accurate, proven.
Real World Evidence Library
Explore our Real World Evidence Library for the latest approaches to answer clinical, regulatory, and commercial questions.
Make treatments stand out. Ensure differentiation lasts.
Enhance commercial effectiveness through precision HCP and patient engagement, backed by unrivalled data, healthcare-grade AI, and domain expertise.
COMMERCIALIZATION OVERVIEW

Data and Information Management
Make better decisions with quality data. Drive business strategies, optimize engagement and uncover new opportunities.
Launch Strategy and Management
Achieve launch excellence with a proven partner. Maximize launch performance and realize full market potential.
Commercial Analytics & Consulting
Gain precision insights for improved commercial effectiveness. Accelerate growth with fact-based decision making.
Commercial Engagement Services
Fuel customer engagement and patient adherence with patient support and commercial outsourcing services.
Established Brands Optimization
Defend market share and maximize value with proven approaches for established brands across global markets.
Service driven. Tech-enabled. Integrated compliance.
Orchestrate your success across the complete compliance lifecycle with best-in-class services and solutions for safety, regulatory, quality and medical information.
COMPLIANCE OVERVIEW

Safety Pharmacovigilance
Pharmacovigilance powered by human expertise and advanced technology to deliver speed, accuracy, and global compliance.
Regulatory Compliance
Regulatory solutions blending human expertise and technology to cut complexity, cost, and risk across the lifecycle.
Quality Compliance
Break down quality and compliance silos with integrated software and services across the full product lifecycle.
Medical Information
Medical information delivered by skilled professionals and innovative technology for quality, scalable support worldwide.
Commercial Compliance
Experienced teams and advanced technology deliver scalable, transparent, and reliable commercial compliance.
Harness technology for a healthier world.
IQVIA Technologies helps life sciences accelerate innovation to make a greater impact on human health. Our transformative technologies harness intelligence, integrate industry leading data and analytics, and use advanced AI/ML capabilities to unleash business potential faster.
TECHNOLOGIES OVERVIEW

Site Suite
Increase transparency, improve communications and reduce administrative burden of your clinical trials from start to finish.
Patient Suite
Simplify trials with a solution that improves operations, elevates experience, and drives better patient outcomes.
Clinical Trial Financial Suite
AI-powered platform unifying budgeting, contracting, forecasting, and payments for streamlined clinical trial financial management.
Safety Suite
Streamline pharmacovigilance with AI-powered automation, global expertise, and real-time safety insights.
Quality Management and Regulatory Suite
Streamline global QA/RA processes with AI-enabled QMS & RIM for compliance, efficiency, and faster market access.
Partner Programs
Join our partner ecosystem to build alliances, drive business value, and create mutually beneficial relationships.
Technology Insights
Check out the latest tech-related resources, thought leadership and best practices for insights that matter most in Life Sciences.
Commercial Compliance
Work with compliance experts to create and manage processes ensuring global regulation compliance.
The IQVIA difference
CLINICAL PRODUCTS
Planning Suite
Grant Plan
Site Suite
Clinical Trial Payments
Investigator Site Portal
One Home for Sites
Patient Engagement Suite
Electronic Clinical Outcome Assessment (eCOA)
Interactive Response Technology (IRT)
Clinical Data Analytics Solutions
COMMERCIAL PRODUCTS
Information Management
Launch Strategy & Management
Pricing & Market Access
Brand Strategy & Management
Promotional Engagement
Patient Insights for HCP Engagement
Alerts & Triggers
Orchestrated Analytics
Next Best Action
Patient Engagement and Support
COMPLIANCE, SAFETY, REG PRODUCTS
IQVIA Vigilance Platform
Commercial Compliance
Regulatory Compliance
SmartSolve® RIM
Quality Compliance
SmartSolve eQMS
Safety & Pharmacovigilance
REAL WORLD PRODUCTS
Real World & Healthcare Data
Health Data Apps & AI
Analytics Research Accelerator
Expert Ecosystem
AI Patient & HCP Profiling- Commercial
AI Patient & Provider Profiling- Med Affairs
AI Patient & HCP Profiling- Healthcare
Natural Language Processing
Market Access Insights
Direct-to-Patient Research
Innovation at IQVIA
At IQVIA our foundation is built on innovation. We are committed to finding new and smarter approaches to solving customer challenges. Explore some of the latest ways we can help drive your results.

IQVIA AI
We’ve developed a novel approach to AI specifically for life sciences to ensure trust, transparency, and transformational results.
Explore IQVIA AI

Collaborating with NVIDIA to launch AI agents
IQVIA’s new custom-built AI agents using NVIDIA technology are designed to enhance workflows and accelerate insights for life sciences.
Read the press release

Protocol Design Optimization
Transform clinical trials with data-driven insights and patient-centric strategies to create smarter protocols, reduce risk, and accelerate study success."
Explore Protocol Design Optimization

IQVIA Medical Reasoning LLM
“Med-R1 8B” is built to empower healthcare and life science organizations, enhance decision-making, and ultimately transform patient outcomes.
Explore IQVIA Medical Reasoning
BLOGS, WHITE PAPERS & CASE STUDIES
Explore our library of insights, thought leadership, and the latest topics & trends in healthcare.
DISCOVER INSIGHTS
THE IQVIA INSTITUTE
An in-depth exploration of the global healthcare ecosystem with timely research, insightful analysis, and scientific expertise.
SEE LATEST REPORTS

WE'RE HIRING
At IQVIA your potential has no limits. We thrive on bold ideas and fearless innovation. Join us in reimagining what’s possible.
VIEW ROLES
Explore IQVIA by region
As a global community, IQVIA continuously invests and commits to advancing human health.
Americas
Europe
Asia & Oceania
Middle East & Africa

Regional Thought Leadership
SOLUTIONS
Research & Development
LIFE SCIENCE SEGMENTS
Consumer Health
Emerging Biopharma
Generics
MedTech
Pharmaceutical Manufacturers
HEALTHCARE SEGMENTS
Information Partner Services
Financial Institutions
Global Health
Government
Patient Associations
Payers
Providers
Therapeutic and Specialty Areas
Therapeutic and Specialty Centers of Excellence offer scientific expertise, therapeutic insights and clinical trial experience to expedite new therapies for patients.
LEARN MORE
Therapeutic and Specialty Areas
Allergy & Respiratory
Biosimilars
Cardiovascular
Cell & Gene Therapy
Central Nervous System
Dermatology
Early Phase Clinical Development
Endocrinology
GI & Hepatology
Infectious Diseases & Vaccines
Nephrology
Obesity
Oncology & Hematology
Ophthalmology
Pediatrics
Rare Diseases
Reproductive Health
Rheumatology
Harness the power to transform clinical development
Reimagine clinical development by intelligently connecting data, technology, and analytics to optimize your trials. The result? Faster decision making and reduced risk so you can deliver life-changing therapies faster.
Research & Development Overview

Early Phase Clinical Trials
Track the impact of new drugs and establish proof of concept faster with innovative early clinical development strategies.
Phase IIb/III Trials
Advanced AI and technology help you navigate regulatory complexity, optimize enrollment strategies and drive patient engagement.
Patient & Site Centric Solutions
Support patients and trial sites with tools and services that improve recruitment, engagement, and study execution.
Functional Services
Agile, data-driven trial solutions that connect people, processes, and technology for faster, smarter R&D delivery.
Global Laboratories
Specialty and central lab solutions backed by scientific rigor and expertise in biomarkers and antibody drug discovery.
R&D Consulting
Optimize your R&D strategy, reduce risk and ensure compliance with data, analytics, AI-powered technology and expertise.
Real World Evidence. Real Confidence. Real Results.
Generate and disseminate evidence that answers crucial clinical, regulatory and commercial questions, enabling you to drive smarter decisions and meet your stakeholder needs with confidence.
REAL WORLD EVIDENCE OVERVIEW

Real World & Health Data Sets
Global real world data exploration at your fingertips enables critical insights across the product lifecycle.
Medical Affairs
Access end-to-end Medical Affairs solutions to plan, generate, and disseminate trustworthy medical evidence.
IQVIA Health Communications Group
Deliver communications that engages stakeholders, advances healthcare and improves lives.
Health Data Apps & AI
Streamline research process and gain access to real world data discovery with the largest agnostic health data catalog.
Health Data Transformation
Accelerate disparate health information into insight-rich, value-driving data transformation at scale.
Study Design
Identify the right study design to answer your research questions with unparalleled data, technology, AI and analytics.
Evidence Networks
Access global evidence networks to connect data, technology, and analytics for broader study options and collaboration.
Health Economics & Value
Generate the evidence you need to go from research to market, overcome regulatory hurdles, or support payer approval.
Regulatory and Safety
Explore options with innovative RWE study approaches to prove your product’s safety and effectiveness.
Natural Language Processing
Extract insights at scale from unstructured text with IQVIA’s advanced NLP platform—fast, accurate, proven.
Real World Evidence Library
Explore our Real World Evidence Library for the latest approaches to answer clinical, regulatory, and commercial questions.
Make treatments stand out. Ensure differentiation lasts.
Enhance commercial effectiveness through precision HCP and patient engagement, backed by unrivalled data, healthcare-grade AI, and domain expertise.
COMMERCIALIZATION OVERVIEW

Data and Information Management
Make better decisions with quality data. Drive business strategies, optimize engagement and uncover new opportunities.
Launch Strategy and Management
Achieve launch excellence with a proven partner. Maximize launch performance and realize full market potential.
Commercial Analytics & Consulting
Gain precision insights for improved commercial effectiveness. Accelerate growth with fact-based decision making.
Commercial Engagement Services
Fuel customer engagement and patient adherence with patient support and commercial outsourcing services.
Established Brands Optimization
Defend market share and maximize value with proven approaches for established brands across global markets.
Service driven. Tech-enabled. Integrated compliance.
Orchestrate your success across the complete compliance lifecycle with best-in-class services and solutions for safety, regulatory, quality and medical information.
COMPLIANCE OVERVIEW

Safety Pharmacovigilance
Pharmacovigilance powered by human expertise and advanced technology to deliver speed, accuracy, and global compliance.
Regulatory Compliance
Regulatory solutions blending human expertise and technology to cut complexity, cost, and risk across the lifecycle.
Quality Compliance
Break down quality and compliance silos with integrated software and services across the full product lifecycle.
Medical Information
Medical information delivered by skilled professionals and innovative technology for quality, scalable support worldwide.
Commercial Compliance
Experienced teams and advanced technology deliver scalable, transparent, and reliable commercial compliance.
Harness technology for a healthier world.
IQVIA Technologies helps life sciences accelerate innovation to make a greater impact on human health. Our transformative technologies harness intelligence, integrate industry leading data and analytics, and use advanced AI/ML capabilities to unleash business potential faster.
TECHNOLOGIES OVERVIEW

Site Suite
Increase transparency, improve communications and reduce administrative burden of your clinical trials from start to finish.
Patient Suite
Simplify trials with a solution that improves operations, elevates experience, and drives better patient outcomes.
Clinical Trial Financial Suite
AI-powered platform unifying budgeting, contracting, forecasting, and payments for streamlined clinical trial financial management.
Safety Suite
Streamline pharmacovigilance with AI-powered automation, global expertise, and real-time safety insights.
Quality Management and Regulatory Suite
Streamline global QA/RA processes with AI-enabled QMS & RIM for compliance, efficiency, and faster market access.
Partner Programs
Join our partner ecosystem to build alliances, drive business value, and create mutually beneficial relationships.
Technology Insights
Check out the latest tech-related resources, thought leadership and best practices for insights that matter most in Life Sciences.
Commercial Compliance
Work with compliance experts to create and manage processes ensuring global regulation compliance.
The IQVIA difference
CLINICAL PRODUCTS
Planning Suite
Grant Plan
Site Suite
Clinical Trial Payments
Investigator Site Portal
One Home for Sites
Patient Engagement Suite
Electronic Clinical Outcome Assessment (eCOA)
Interactive Response Technology (IRT)
Clinical Data Analytics Solutions
COMMERCIAL PRODUCTS
Information Management
Launch Strategy & Management
Pricing & Market Access
Brand Strategy & Management
Promotional Engagement
Patient Insights for HCP Engagement
Alerts & Triggers
Orchestrated Analytics
Next Best Action
Patient Engagement and Support
COMPLIANCE, SAFETY, REG PRODUCTS
IQVIA Vigilance Platform
Commercial Compliance
Regulatory Compliance
SmartSolve® RIM
Quality Compliance
SmartSolve eQMS
Safety & Pharmacovigilance
REAL WORLD PRODUCTS
Real World & Healthcare Data
Health Data Apps & AI
Analytics Research Accelerator
Expert Ecosystem
AI Patient & HCP Profiling- Commercial
AI Patient & Provider Profiling- Med Affairs
AI Patient & HCP Profiling- Healthcare
Natural Language Processing
Market Access Insights
Direct-to-Patient Research
Innovation at IQVIA
At IQVIA our foundation is built on innovation. We are committed to finding new and smarter approaches to solving customer challenges. Explore some of the latest ways we can help drive your results.

IQVIA AI
We’ve developed a novel approach to AI specifically for life sciences to ensure trust, transparency, and transformational results.
Explore IQVIA AI

Collaborating with NVIDIA to launch AI agents
IQVIA’s new custom-built AI agents using NVIDIA technology are designed to enhance workflows and accelerate insights for life sciences.
Read the press release

Protocol Design Optimization
Transform clinical trials with data-driven insights and patient-centric strategies to create smarter protocols, reduce risk, and accelerate study success."
Explore Protocol Design Optimization

IQVIA Medical Reasoning LLM
“Med-R1 8B” is built to empower healthcare and life science organizations, enhance decision-making, and ultimately transform patient outcomes.
Explore IQVIA Medical Reasoning
BLOGS, WHITE PAPERS & CASE STUDIES
Explore our library of insights, thought leadership, and the latest topics & trends in healthcare.
DISCOVER INSIGHTS
THE IQVIA INSTITUTE
An in-depth exploration of the global healthcare ecosystem with timely research, insightful analysis, and scientific expertise.
SEE LATEST REPORTS

WE'RE HIRING
At IQVIA your potential has no limits. We thrive on bold ideas and fearless innovation. Join us in reimagining what’s possible.
VIEW ROLES
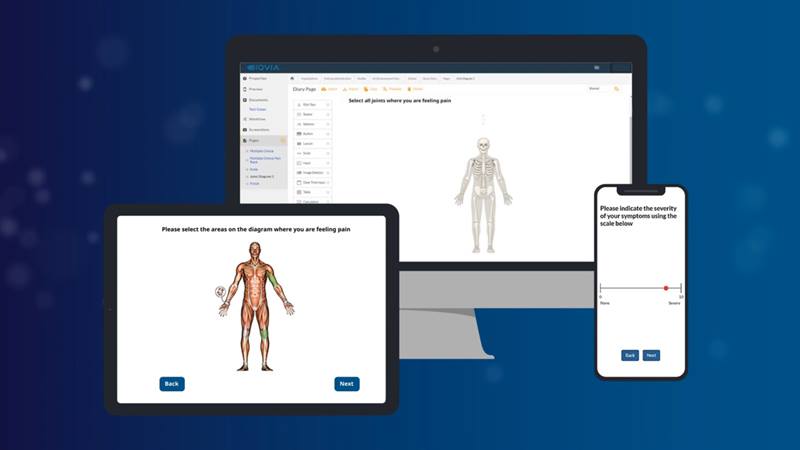
IQVIA eCOA delivers unparalleled data quality, integrity and patient compliance to optimize your data collection. Experience an eCOA platform that provides accelerated pathways to market with transformative decisions fueled by real-time insights.


IQVIA eCOA captures data in real time to accelerate decision-making.
Our eCOA solution does all the heavy lifting to ensure your primary endpoints are achieved. With real-time data capture, autogenerated regulatory and project documentation and streamlined workflows, IQVIA eCOA will help you reduce implementation timelines by up to 75%.
An eCOA solution designed for you, built for your patients.
IQVIA eCOA is purpose-built to support the evolving demands of modern clinical research, combining scientific credibility, global scalability and advanced digital innovation. Our eCOA solution enables confident decision-making by delivering high-quality, regulatory-ready data through a seamless and patient-centric experience. With deep therapeutic expertise and a proven global footprint, IQVIA eCOA accelerates study timelines, enhances data integrity and ensures operational excellence across every phase of your trial. Explore how below:
Diary Creation
Intuitive eCOA design at your fingertips with IQVIA Sculptor
IQVIA Sculptor simplifies the diary creation process for sponsors by offering a highly visual, drag-and-drop interface that streamlines the design of eCOA instruments. Instead of relying on complex programming, lengthy specification documents and lots of back and forth with designers, sponsors can quickly build, customize and preview patient diaries in real time. Sculptor ensures consistency with regulatory and protocol requirements while reducing development timelines and minimizing costly rework.
Its collaborative features also allow cross-functional teams to review and iterate on content within a single platform, enhancing transparency and accelerating study startup. By making diary creation more intuitive and efficient, Sculptor empowers sponsors to focus on study outcomes rather than technical hurdles.
See IQVIA eCOA in action
IQVIA eCOA Demo- Sculptor Portal
Patient-Centric Experience
The IQVIA Scribe App: Designed for the patient experience
The IQVIA eCOA solution is designed with a strong emphasis on patient-centricity, and the patient facing IQVIA Scribe app exemplifies this approach. Scribe streamlines the data collection process by offering an intuitive, user-friendly interface that empowers patients to easily report their health outcomes in real time, no matter what they’re doing. The app supports multiple languages and accessibility features, ensuring inclusivity across multiple patient populations. By minimizing technical barriers and integrating seamlessly with patients’ daily routines, Scribe enhances engagement and compliance, ultimately improving data quality and the overall clinical trial experience. Its offline capabilities and secure data handling further reinforce trust and convenience, making it a vital tool in modern, patient-focused research.

Blog
IQVIA wins the Fierce CRO Award for Innovative Approaches to Patient-Centric Research
IQVIA has been honored with the Fierce CRO Award for its work in patient-centric research. By integrating innovative technologies and deeply understanding patient needs, IQVIA is redefining how clinical trials are designed and executed. This approach prioritizes accessibility, engagement and real-world relevance, ensuring that patients are active partners in the research journey. This recognition underscores IQVIA’s leadership in shaping a more inclusive and effective future for clinical development. Read more about the award, what winning means to IQVIA and where our patient focused products are going in the future in this blog.
Efficiency and Globalization
Efficiency Meets Scale: The Power of the IQVIA eCOA Library
The IQVIA eCOA Library is a powerful resource designed to accelerate study startup and reduce complexity for sponsors by providing access to a curated collection of pre-validated, protocol-ready eCOA instruments. With standardized formats and proven configurations, sponsors can quickly identify and deploy the right assessments for their trials, minimizing customization and reducing time spent on design and validation. The library supports regulatory compliance and global scalability, ensuring consistency across studies while maintaining flexibility for specific therapeutic areas. By leveraging the eCOA Library, sponsors gain a faster, more efficient path to high-quality data collection and improved patient engagement.

Case study
Custom eCOA library reduces oncology program timelines by 50%
Discover how a large oncology sponsor created a customized eCOA library to collect accurate, daily reported patient data across 13 countries. There was no room for error to ensure each input could ladder up into one resolution.
IQVIA eCOA enabled the sponsor to create eight new COA instruments, manage 27 languages and obtain all the necessary validations and licenses to accelerate the successful outcome of this vital oncology trial.
75% faster trial start up
When using an assessment that is already in an eCOA library
> 600
Validated and approved assessments in the library, with more added all the time
>2,500
Pre-built eCOA forms in the library
50
Collaborations with instrument owners
Increased Compliance
IQVIA eCOA: Driving Unmatched Patient Compliance Through Smart, Inclusive Technology
The IQVIA eCOA solution delivers industry-leading compliance rates, up to 95% in studies using automated data monitoring, by combining intuitive design, real-time data capture and inclusive technology. Patients can use their own devices or provisioned ones, with support for online and offline reporting, caregiver input and accessibility features like larger screens and fonts. Automated prompts and alerts keep participants engaged, while centralized updates ensure seamless mid-study changes. With the highest compliance rates and the broadest support for diverse populations, IQVIA eCOA empowers sponsors to collect cleaner data, reduce missing entries and enhance trial outcomes.

Ensuring your BYOD strategy meets the rigor of regulators
Can patients reliably complete eCOAs on their own devices?
In today’s patient-centric trials, eCOAs empower participants to share their experiences from anywhere—no clinic visit required. But as sponsors explore Bring Your Own Device (BYOD) strategies to reduce burden and boost compliance, one question looms large: will regulators approve it? Discover how IQVIA helps sponsors navigate BYOD implementation with confidence, ensuring data quality, regulatory readiness and a seamless patient experience.
Amplifying the Patient Voice
Turning Up the Volume on Patient Voice in Clinical Trials
IQVIA’s eCOA platform is designed to elevate the patient experience by making it easier, more intuitive and more inclusive for participants to share their treatment journeys. With secure, user-friendly apps that work across personal and provisioned devices, whether online or offline, patients can report outcomes anytime, anywhere. Automated prompts and alerts help ensure assessments are completed on time, while accessibility features like larger fonts and caregiver login options support diverse needs. By enabling real-time, high-quality data collection directly from the patient, IQVIA eCOA ensures that every voice is heard, every experience is captured and every insight drives better trial outcomes.

Tailored solutions
- For patient populations requiring caregiver reporting or assistance, IQVIA eCOA offers caregiver login functionality.
- IQVIA eCOA partners with IQVIA’s Patient Centered Solutions team to ensure that the clinical outcome assessment strategy aligns with targeted patient population and study objectives.
- For patients experiencing vision impairment, IQVIA eCOA provides bigger screens and tablets with larger font sizes.

Easy-to-use-app
- Preprogrammed logic and edit checks help patients navigate questionnaires successfully.
- Notifications and alerts prompt patients to complete eDiaries, improving compliance rates.

Bring your own device
- IQVIA eCOA allows patients to securely use devices they already own, which reduces costs and patient burden while increasing patient compliance.
- IQVIA eCOA provisions devices where needed, including iPad devices and other tablets if smaller devices are not ideal for some patients.
- IQVIA eCOA provides device provisioning services that expand access to trial participation to socioeconomically and geographically diverse populations.
- Backup devices are available if patients’ primary devices fail.

Patient compliance
- IQVIA eCOA technology works seamlessly with patients’ daily routines to make capturing data and recording important experiences easy.
- An intuitive user interface offers a consistent experience for patients whether they use it online or offline.
- Compliance statistics help clinicians see how well patients are keeping up with reporting.

Robust training
Along with a show of appreciation, IQVIA eCOA supports trial participants with easy-to-follow training that also helps to mitigate technology hesitancy.

Therapeutic alignment
IQVIA eCOA configures each assessment experience to align with specific therapeutic-area requirements, including:
- Device type, display font size and display color
- Navigation features and response options
- Assessment breaks and resumption of functionality
DISCOVER WHY PALVELLA THERAPEUTICS QUICKLY BENEFITED FROM DEPLOYING IQVIA eCOA
IQVIA and Palvella Therapeutics: Why IQVIA?
IQVIA accelerates performance excellence in obesity trials
IQVIA helped accelerate performance excellence in obesity trials by combining scientific expertise, digital technology, including eCOA solutions and end-to-end support. The platform achieved over 90% patient compliance, improved data quality by 50% and reduced setup timelines by up to 75%, enabling sponsors to run faster, more efficient, and regulator-ready studies.
In response to the rapidly growing obesity drug market, IQVIA delivered a comprehensive solution that streamlined trial execution and enhanced data integrity. By integrating scientific strategy, preapproved obesity assessments within eCOA and connected device support, IQVIA enabled sponsors to design and implement trials with speed and precision. The platform’s automation features, such as real-time alerts triggered by glucometer readings, minimized patient burden and ensured timely, accurate data capture. IQVIA’s agile ecosystem supported adaptive trial designs and faster database lock timelines, driving deeper insights and proactive decision-making. Over the past five years, IQVIA has supported more than 80 obesity trials across 45 countries, enrolling over 60,000 patients. The result: a one-stop shop for high-performance, compliant obesity trials that deliver measurable impact.
Challenge
Challenge: Navigating Complexity in a Rapidly Growing Obesity Market
With the global obesity drug market surpassing $30 billion in 2024, sponsors face mounting pressure to run fast, efficient and regulator-ready trials. They must navigate complex trial designs, ensure high-quality data collection through eCOAs and adapt quickly to protocol changes—all while maintaining scientific rigor and compliance.
The obesity drug market is expanding rapidly, creating intense demand for clinical trials that are both scientifically sound and operationally agile. Sponsors must manage multifaceted challenges, including designing effective trial strategies, selecting appropriate outcome assessments and ensuring data accuracy and timeliness. eCOAs play a critical role, but they must be flexible enough to accommodate protocol changes and deliver regulator-ready data. Speed and innovation are essential, as sponsors strive to differentiate their products and meet evolving regulatory expectations. The complexity of obesity trials—often involving comorbidities and diverse endpoints—requires a strategic, integrated approach to trial design and execution. IQVIA’s solution addresses these needs by combining scientific expertise, digital technology and end-to-end support to streamline the entire trial lifecycle.
Solution
End-to-End Innovation for Smarter Obesity Trial Execution
IQVIA delivered an integrated, end-to-end solution for obesity trials, combining scientific strategy, eCOA technology and connected device support. Their agile platform accelerated setup timelines by up to 75%, improved data quality by 50% and achieved over 90% patient compliance—ensuring trials were efficient, compliant and regulator-ready.
IQVIA’s solution brought together scientific expertise, digital innovation and operational support to streamline obesity trial execution. Their team of seasoned advisors guided sponsors through endpoint strategy, instrument selection and regulatory alignment, ensuring scientifically sound and compliant trial designs. The eCOA platform was built with agility in mind, enabling rapid setup and seamless integration with connected devices like glucometers, which automatically triggered relevant assessments based on patient data. IQVIA’s preapproved library of obesity-related assessments further accelerated study builds. The platform’s automation and real-time monitoring capabilities reduced patient burden, improved data accuracy and supported adaptive trial designs. With over 80 obesity trials supported across 45 countries, IQVIA’s comprehensive approach delivered measurable improvements in trial performance and data quality.
Results
Driving Excellence
in Obesity
Research
IQVIA’s comprehensive solution delivered measurable impact across obesity trials, achieving over 90% patient compliance and improving data quality by 50%. Their agile platform expedited COA strategies by up to 40% and reduced eCOA setup timelines by as much as 75%, supporting faster, more efficient trial execution.
IQVIA’s eCOA platform significantly enhanced trial performance in obesity studies, achieving over 90% patient compliance with diaries and improving data quality by 50%. The solution accelerated COA measurement strategies by up to 40% and reduced eCOA setup timelines by up to 75%, enabling faster trial starts and more efficient execution. Integrated connected devices, such as glucometers, automatically triggered relevant assessments based on patient data, reducing manual entry and ensuring timely event capture. An automated dashboard monitored trends and risks, allowing for proactive issue resolution. The platform’s agility supported adaptive trial designs and faster database lock timelines, streamlining final endpoint analysis. Over the past five years, IQVIA has supported more than 80 obesity trials across 45 countries, enrolling over 60,000 patients at more than 2,000 sites.
Read the full case study now

Blog
eCOAs support key study endpoints while enhancing site and patient experience





