

The rapid rise in healthcare information is driving increased scrutiny of its collection and utilization and growing challenges for companies to interact effectively with the healthcare community.
Perhaps one of the greatest impact of all is anticipated from the General Data Protection Regulation (GDPR). Now in place and setting new standards for data protection in Europe. The seven core GDPR impact areas to be adhered to by pharma are:
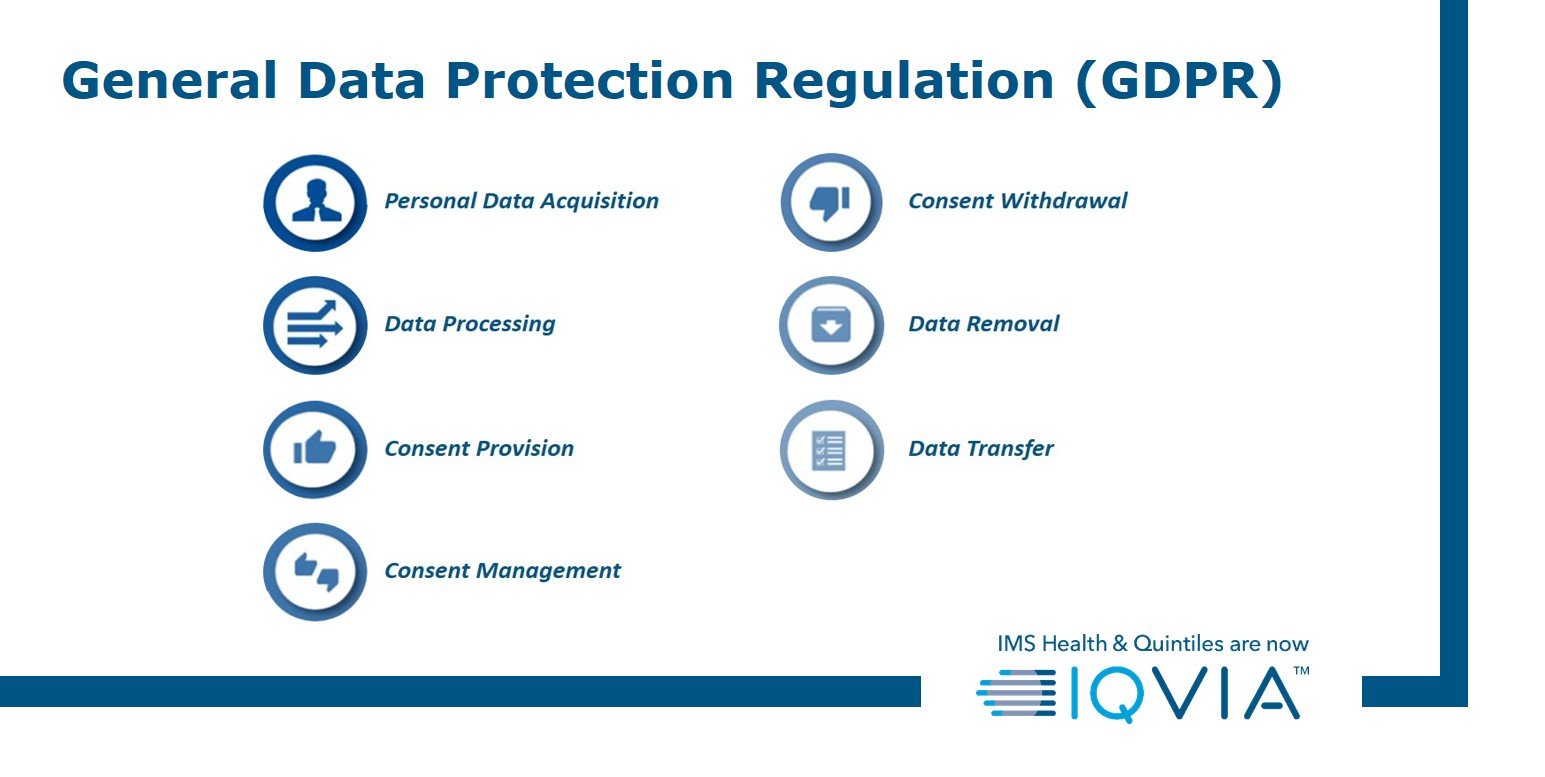
Furthermore, among the latest regulations governing disclosure and transparency is the Belgian Sunshine Act of 2016. With international scope and covering all transfers of value between the industry and HCPs/HCOs, this adds a new layer of complexity to the wide array of existing anti-corruption laws requiring companies to justify such interactions.
Even as legislation becomes more stringent, there remains a lack of harmonization in terms of what is meant by transparency, beneficiaries, transfers of value, reporting procedures and the type of companies subject to disclosure. Companies must also bear in mind the wide variety of interlocutors, each with their own channels of engagement and risk.
To summarize:

IQVIA can help you navigate this increasingly complex landscape. Our global compliance capabilities with local market impact are powered by a singular industry focus and deep consulting, technology and subject matter expertise. We work with you to turn the challenges of compliance into new opportunities for your HCP/HCO engagement, through:

Our solutions enable you to drive business value by using compliance to: Gain process and cost efficiencies, make smarter business decisions and enable greater predictability in HCP engagement.
For more details on these insights, please contact us.

