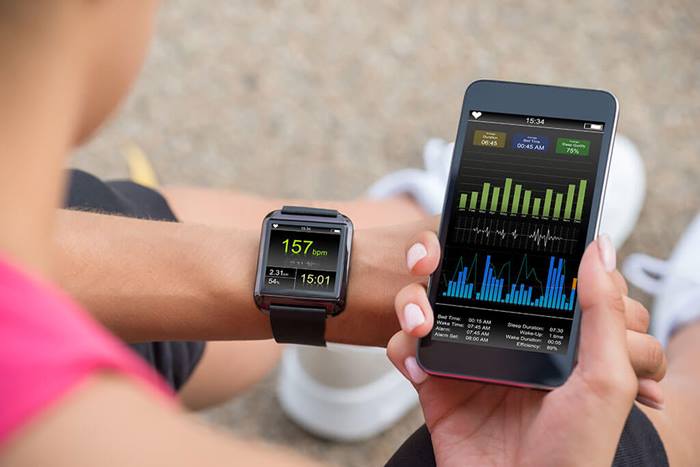
Bring trials directly to patients to improve access and engagement, increase quality and shorten timelines.
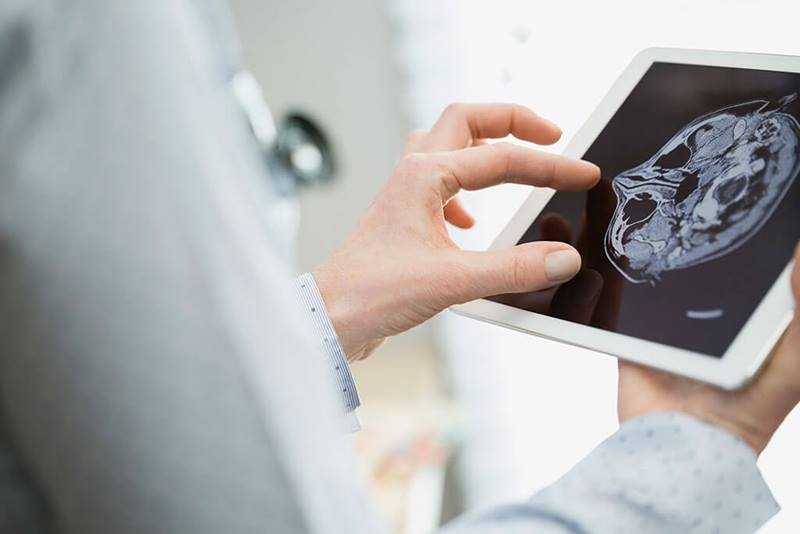

























Applying digital tools and techniques to your business can support a range of strategic initiatives. It can lower operating costs, improve differentiation, mitigate risk, or even open up an entirely new direction. And managing these transitions is handled differently, depending on what is right for the culture and the objective for the business — from hiring a chief digital officer, to infusing an imperative for innovation across the organization.
IQVIA can help life sciences organizations, at every stage of digital maturity, see where applying digital tools, techniques, channels and even treatments can close the gap between data collection and clinical decision-making. Trigger positive changes in care management. Push healthcare further.

The value of digital is helping to do what you need to do to improve your connection with patients, payers and providers in a better, faster, more modern way.



This report reviews the unmet needs of people with diabetes and takes a look at how smart insulin pens can address some of the difficulties of self-management and improve outcomes.
Bring trials directly to patients to improve access and engagement, increase quality and shorten timelines.
Discover unrealized connections and manage your product across the entire lifecycle, from research and development, compliance to launch and commercialization.
See how IQVIA has helped organizations across healthcare evaluate and execute digital health strategies. The integration of digital data, technology and analytics means a new, more powerful ecosystem of real world evidence. Keep reading to learn more.