Discover new approaches to cardiovascular clinical trials to bring game-changing therapies to patients faster.






















Following the initial top-line readout in August – which showed a statistically significant, 20% relative risk reduction in MACE-3 cardiovascular events – the veil has finally been lifted from the landmark SELECT cardiovascular outcomes trial. Novo Nordisk shared further details in its much anticipated presentation of the full data from SELECT at the American Heart Association Scientific Sessions 2023.
So what did we learn?
As a reminder, SELECT enrolled 17,604 adults who were 45 years or older, overweight or obese, without diabetes and had a diagnosis of cardiovascular disease, including prior myocardial infarction, stroke, and/or peripheral artery disease. The trial was designed to evaluate the effect in this population, over a period of up to five years, of a once‐weekly subcutaneously administered dose of 2.4 mg semaglutide (Wegovy) on cardiovascular outcomes compared to placebo.
Highlights from Novo Nordisk’s presentation include (see Figure 1):
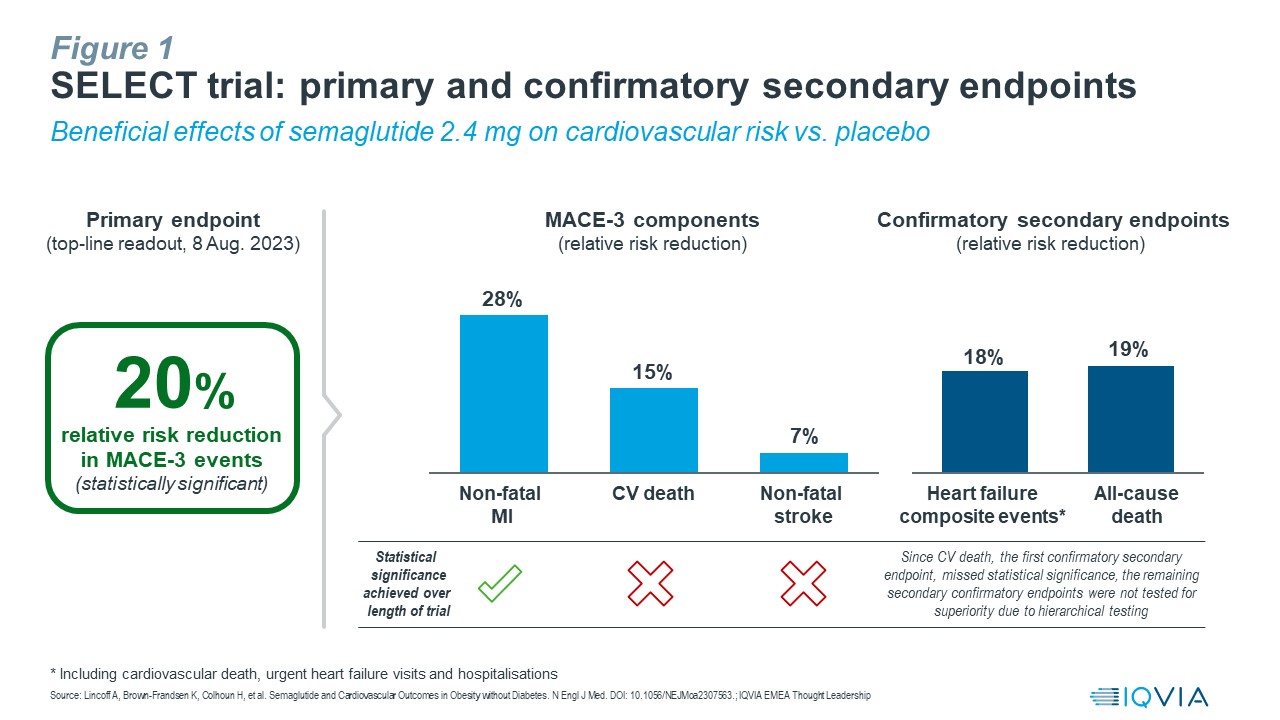
- Analysis of the three components in MACE: Semaglutide 2.4 mg reduced the risk of…
- Non-fatal myocardial infarction by 28%
- Cardiovascular death by 15% (not statistically significant over the length of the trial)
- Non-fatal stroke by 7% (not statistically significant over the length of the trial)
-
Analysis of confirmatory secondary endpoints: Semaglutide 2.4 mg reduced the risk of…
- Composite heart failure events, comprising cardiovascular death, urgent heart failure visits and hospitalisations, by 18%
- Death from any cause by 19%
- Note: Since the result on cardiovascular death, the first confirmatory secondary endpoint, was not statistically significant over the length of the trial, the remaining secondary confirmatory endpoints were not tested for superiority due to hierarchical testing.
- Analysis of supportive secondary endpoints: Semaglutide 2.4 mg was also associated with improvements in multiple cardiovascular risk factors, including waist circumference, glycated haemoglobin level, systolic and diastolic blood pressure, high-sensitivity C-reactive protein and lipids.
- Adverse events: There were no unexpected safety findings, however, gastrointestinal (GI) side effects were a main cause of treatment discontinuation, which occurred in 16.6% of patients in the semaglutide group vs. 8.2% in the placebo group.
- Drivers of cardiovascular benefit: Reduction in MACE-3 events occurred early, soon after treatment initiation, before any significant weight change was achieved. This suggests weight loss alone may not fully explain the observed benefits of semaglutide and that other factors may also be at play, e.g., potential anti-inflammatory effects.
A resounding success for Novo Nordisk
The SELECT results exceed expectations of many analysts and investors, who were hoping to see 12-17% MACE-3 reduction. Clinical audiences are equally impressed with the data, hailing a new era for how obesity is perceived and treated, notwithstanding the missed statistical significance for cardiovascular death and the other secondary confirmatory endpoints not being tested for superiority as a consequence.
Crucially, with SELECT, Novo Nordisk has established the missing link between weight management and saving lives.
These compelling results set Wegovy distinctly apart: To date, there are no approved weight management medications proven to deliver effective weight loss and reducing MACE-3 events, while also being associated with improvements in multiple cardiovascular risk factors. Only Wegovy now has the supporting evidence on relevant outcomes, which Novo Nordisk is set to convert into an expanded label and use with payers to broaden coverage.
This puts pressure on its competitors to generate their own CV outcomes data, with a high bar to clear. However, Wegovy will enjoy a data advantage for the next few years until its competitors manage to catch up with their own outcomes trial readouts, which are not expected until Q4/2027.
It will also be interesting to see whether and how differences in headline figures for weight loss delivered by different therapies translate into CV benefits and, especially, if this would give more potent therapies than Wegovy a competitive advantage.
While SELECT demonstrated compelling outcomes, stakeholders will likely look for further evidence to substantiate the benefits of weight management with Wegovy outside the controlled setting of a clinical trial, e.g., in real world studies to confirm effectiveness and safety in routine medical practice when treating large numbers of patients of greater diversity, or via the SELECT-LIFE extension study, which invites SELECT patients to participate in a 10-year observational follow-up to evaluate long-term benefits.
Looking beyond SELECT, which focused on secondary prevention, the ultimate prize is still to be seized – primary prevention, a huge potential opportunity. However, capturing it will require differently designed, large long-term outcomes trials in much broader patient populations.
Implications of SELECT for the management of cardiovascular risk
Firstly, SELECT has shown that the already known cardioprotective effect of GLP-1 receptor agonists is not limited to patients with diabetes.
Many patients in the trial were also receiving standard-of-care therapies for their underlying cardiovascular disease, with 90.1% of patients taking lipid-lowering medications, 86.2% antiplatelets, 70.2% beta-blockers, 45.0% ACE inhibitors and 29.5% ARBs. SELECT demonstrated that ‘add-on treatment’ with Wegovy clearly provided additional cardiovascular protection. It will therefore be hard to argue against patients of similar profile to the SELECT trial population receiving Wegovy to reduce their overall CV risk.
Furthermore, the SELECT results support the broader case for a weight-centric approach to CV risk management and a change in treatment guidelines. Its premise is an upstream intervention targeting substantial weight reduction to improve key risk factors with the aim to avoid potential downstream complications from multiple co-morbidities, e.g., hypertension, dyslipidaemia, heart failure, kidney disease, NAFLD/NASH or diabetes.
This would establish Wegovy, and possibly other potent anti-obesity therapies, as a backbone of CV risk management, with far-reaching implications beyond a change in clinical practice.
It would reshape the market for treating specific cardio-metabolic conditions, potentially shrinking the downstream commercial opportunity if the CV risk baseline was reset by an upstream intervention focussed on weight management. As a consequence, SELECT has raised the evidence threshold that innovators must clear when targeting conditions such as hypertension, dyslipidaemia or heart failure, including the effect size they need to demonstrate to be clinically meaningful and patient relevant.
Additionally, if CV risk was managed upstream through weight reduction, unmet need due to high residual CV risk would likely manifest itself in specific patient sub-segments and thus require innovators to take a highly targeted approach in both clinical development and commercialisation.
Finally, SELECT’s unambiguous message that weight management saves lives makes a compelling case, and creates an ethical imperative, for payers and policy makers to expand coverage, including revisiting the statutes prohibiting Medicare from covering anti-obesity drugs. No doubt, to guide their deliberations these stakeholders will look closely at both relative and absolute risk reduction to quantify the net value of such potential coverage expansion.
All eyes are now on how payers and policy makers will respond in due course.
* * *
To learn more about the obesity opportunity, please read our obesity deep dive, part of our recent white paper on the broader renaissance of cardiometabolic innovation. Download our white paper here: https://www.iqvia.com/library/white-papers/a-renaissance-for-cardiometabolic-innovation
you may also be interested in
New Demand in an Old Market
How the launch of Mounjaro transformed the GLP-1 market
Transitional Assistance Programs in the GLP-1 Market
How three brands approached a restricted market landscape
Therapeutic Breakthroughs Drive Dealmaking in Endocrine and Metabolic Disorders
IQVIA Pharma Deals
Related solutions
Specialized expertise and customized solutions across 14 therapeutic centers of excellence, including oncology, GI/NASH, pediatrics, neurology and rare diseases.





