Strengthen your portfolio by making more informed decisions and prepare for the impact of new research discoveries.






















- Blogs
- Emerging Treatments Intensify the Battle for Atopic Dermatitis Patient and Sales Share
Atopic dermatitis (AD) is a common disease with significant quality-of-life implications for patients with severe symptoms. As the understanding of AD grows, new but costly treatment options are emerging. IQVIA’s Forecast Link and Patient Link allow the comparison between the number of patients treated with a drug and its actual sales.
AD is the most common type of eczema and affects millions of people globally. Multiple factors are thought to cause the disease including genetics, environmental factors, and an overactive immune system. The disease triggers an attack on the skin causing rough, painful, and itchy patches of skin1. Symptoms usually begin early in childhood and can be severe and long-lasting, seriously affecting a patient’s quality of life.
Topical corticosteroids and emollients have been the mainstay of treatment for AD for many years. Topical emollients are the first line of treatment which create a protective barrier on the skin, help the skin retain moisture and protect it from irritants2. Regular treatment with emollients can help prevent patches of inflammation or flare-ups. During a flare-up, application of an emollient is often followed by application of a topical corticosteroid to suppress the immune response.
IQVIA’s Patient Link can be used to assess the population of patients within a disease and country, the average number of drugs a patient is prescribed and the number of patients prescribed each individual drug. It calculates patient numbers at a drug level and compares the sum of this calculation to independent country-specific patient number sources, adding the useful measure of patient-centered metrics. In Patient Link, a comparison is made between literature-sourced treated patients and total drug-treated patients calculated using demand-based data. The difference between these two values is explained by implied concomitance, or the number of therapies the average patient is taking at the same time. Importantly, most patients treated for AD require more than one drug to control their symptoms, and as a result, individual patients are counted more than once when using the drug-treated patients metric. The patient share of individual products or classes of products can then be calculated by dividing the drug-treated patients obtained from sales data by the treated patients estimated from literature sources.
According to Patient Link, in the USA in 2021 over 100% of drug-treated patients were treated with hydrocortisone, a common corticosteroid, and over 200% of drug-treated patients were treated with a topical corticosteroid of some kind. This can be explained by the numerous corticosteroids that are available with varying strengths and formulations and thus patients may be prescribed more than one depending on the area of body affected and the severity of the outbreak. On average, Patient Link data estimates that AD patients in the 7 major markets (7MM) use 3.3 prescription drugs to treat their condition. Other widely used therapy classes for AD include antibiotics to treat bacterial skin infections stemming from open sores or cracks in the skin and antihistamines to manage itch.
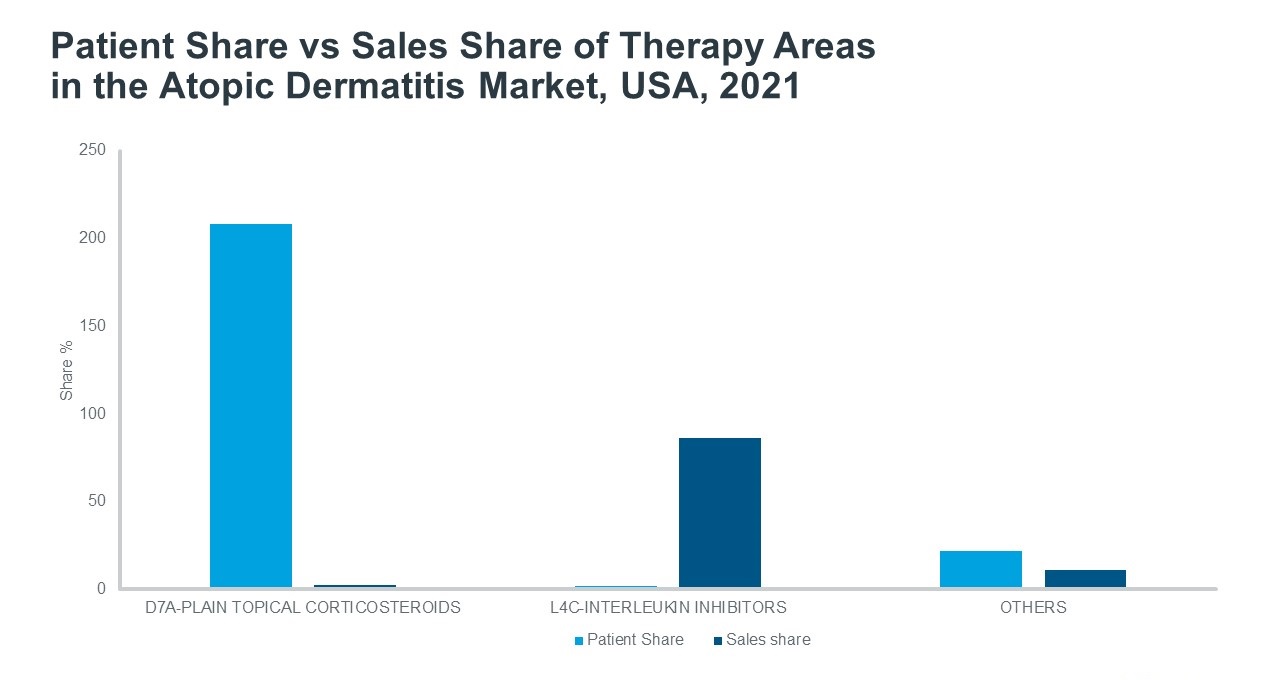
Source: IQVIA Forecast and Patient Link, July 2022
However, for some patients, symptoms are not controlled adequately with topical regimens, and this is where newer therapies are gaining value and patient share. The first biologic medicine to treat AD was approved in the USA for adults in 2017 and has since received multiple further approvals to include all ages above 6 months for patients where the disease is not adequately controlled with topical prescriptions. The biologic therapy is administered subcutaneously and acts by blocking interleukin (IL)-4 and IL-13, two cytokines that cause inflammation. IQVIA Forecast Link predicts sales will reach $5.8 bn by 2031 in the USA and the drug is expected to account for a 61% share of the total atopic dermatitis market in value terms despite only being used to treat around 3% of patients in the same year. Only a very small number of patients from a large population pool get access to these treatments due to the significant cost compared to older treatment options. The approval of another IL-13 inhibitor in 2021 and expected approvals of two additional IL inhibitors in 2023 and 2025 further adds to the rapid growth of sales forecast for this mechanism of action between 2021 to 2031.
In the 7MM, Forecast Link forecasts the use of topical corticosteroids will remain stable from 2017 to 2031, whilst the declining use of emollients is expected to continue. The introduction and rapid increase in the use of janus kinase (JAK) inhibitors drives patient share growth for this disease. JAK inhibitors act by blocking the JAK-STAT pathway and provide the benefit of inhibiting multiple inflammatory cytokines, rather than just a select one or two compared to IL-inhibitors. The first JAK inhibitor for the treatment of AD in the USA was approved in September 2021 and is administered topically. This was followed by the approval of an orally-administered JAK inhibitor in January 2022.The broader mechanism of action, ease of administration and rapid onset of action is likely the reason behind the huge patient share JAK inhibitors are expected to capture by 2031, despite the less favourable safety profile when compared to IL inhibitors. JAK and interleukin inhibitor launches are expected to compete for the comparatively small patient population able to receive these expensive treatments indicated for more severe patients. Therefore, Forecast Link models some erosion of existing treatments, and share shift to future launches.
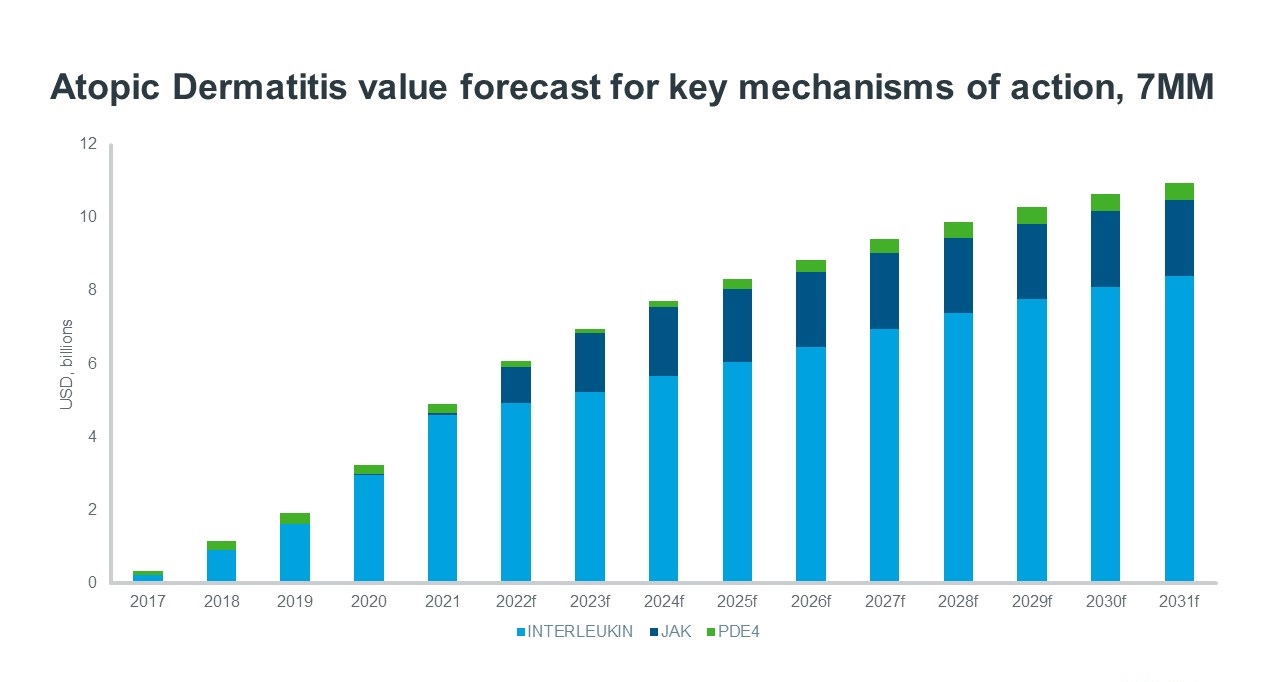
Source: IQVIA Forecast and Patient Link, July 2022
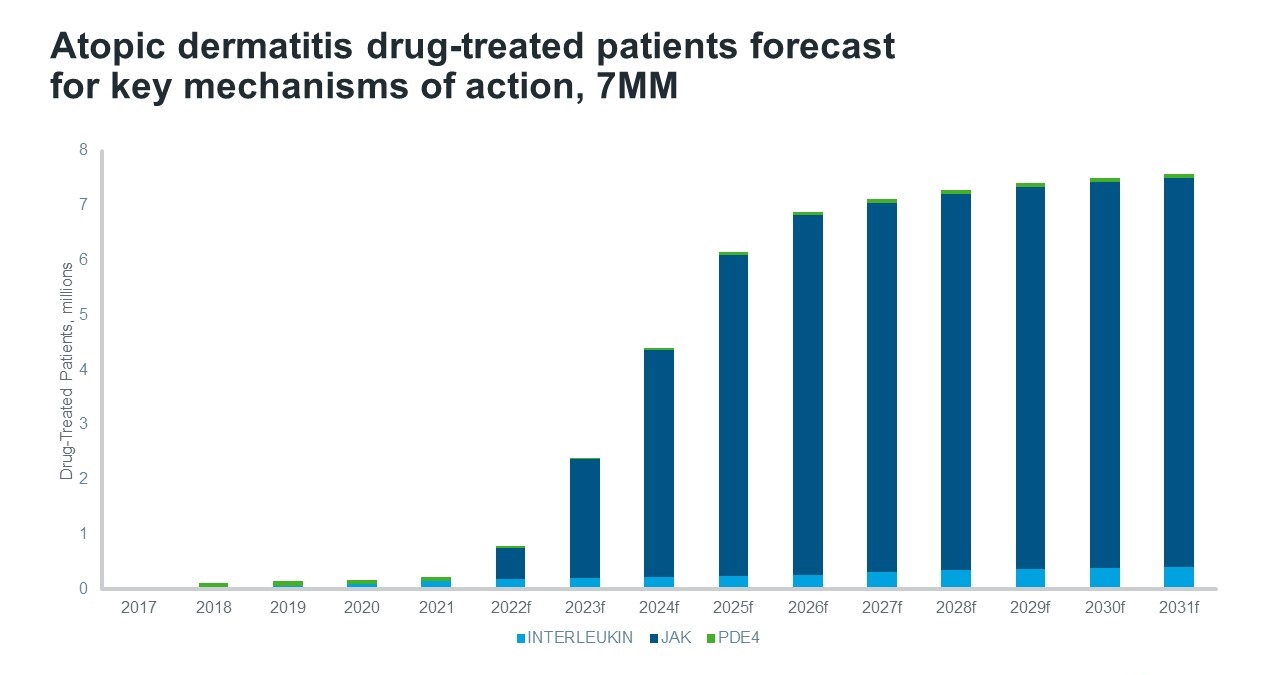
Source: IQVIA Forecast and Patient Link, July 2022
There is still significant unmet need in the treatment of AD, but as knowledge of the pathogenesis of the disease improves, drug development continues to advance. Other potential new treatments currently in Phase III development include tradiptant – - a novel oral neurokinin (NK)-1 inhibitor, lebrikizumab – an IL-13 inhibitor and tapinarof – a topical aryl hydrocarbon receptor agonist. Over the next decade, however, JAK inhibitors look set to win the battle for patient share whilst IL inhibitors are forecast to win the value share among emerging treatments for AD.
References:
- Buys, L.M. (2007). Treatment Options for Atopic Dermatitis. American Family Physician, [online] 75(4), pp.523–528. Available at: https://www.aafp.org/pubs/afp/issues/2007/0215/p523.html [Accessed 19 Jul. 2022].
- Hon, K.L., Kung, J.S.C., Ng, W.G.G. and Leung, T.F. (2018). Emollient treatment of atopic dermatitis: latest evidence and clinical considerations. Drugs in Context, [online] 7, pp.1–14. doi:10.7573/dic.212530.
For more information on brand and country specific forecasts and patient numbers in any disease, please contact IQVIA and request a demonstration of Forecast Link and Patient Link. These are integrated online tools containing 5 years historical and 10 years of forecast data in 73 countries, for 10,000 drugs and over 600 diseases, providing unrivalled breadth of coverage and is updated quarterly.
You may also be interested in
Related solutions
Be proactive about growing your brand using the latest in data, analytics, and domain expertise.





