Study Start-Up
Give your trials a head start that lasts
Orchestrated Study Start-Up speeds site activation and keeps sites engaged throughout the entire trial.






















Orchestrated Study Start-Up speeds site activation and keeps sites engaged throughout the entire trial.



Orchestrated to engage sites throughout the entire study
Study start-up (SSU) is so much more than a one-time document management exercise. It’s a global, strategic operation that can get new drugs approved faster – and it’s ripe for innovation – from Site Selection to Site Activation and Site Training.
Many SSU tech solutions deployed by sponsors don’t deliver the results promised because they add burden without benefits to clinical research sites. The result? Site staff simply avoid using them.
When that happens, document exchange and tracking falls back to paper, email and Excel formats – with CRAs holding the processes together. The tools that were supposed to solve a problem become part of the problem – and consume precious clinical trial budget.

Automate user activities and track start-up data in your system of record with bi-directional CTMS orchestration.
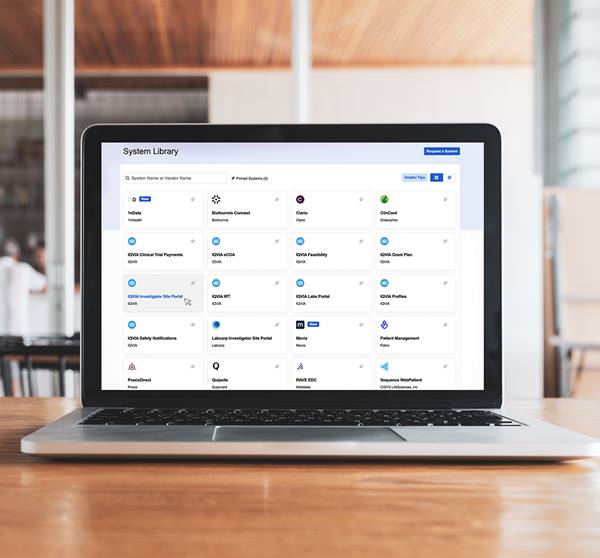
Create consistent start-up processes with global templates for use across all trials and enable study teams to set up even large trials in a day
Select templates from the Global Catalog to configure start-up activities for varying study requirements by therapeutic area or countries chosen
Guide study team and site users through document collection and other site-activation steps using action icons, task-based filters, and notifications
Prepare and track regulatory and health authority submissions, including amendments and resubmissions
Transfer approved country and site documents to your eTMF system of choice, including required metadata values
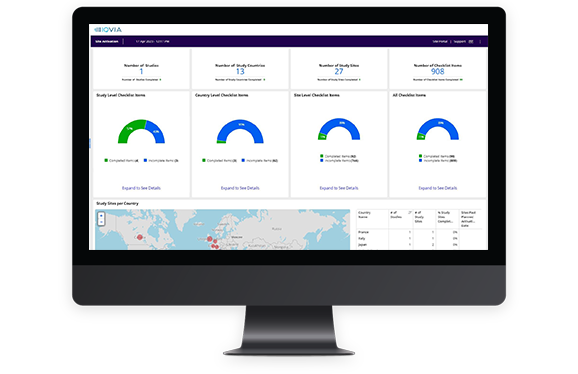
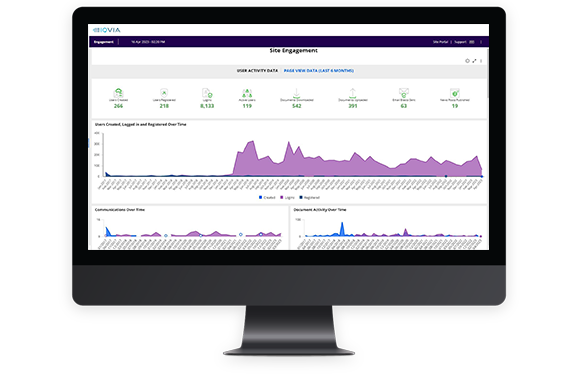
Cross-study dashboards on site activation, training, and engagement metrics, available at the study, country, and site levels
Expedite site selection decisions with built-in workflows that automate CDA acceptance and increase survey responses
Get to first patient/first visit faster and eliminate site frustrations by automating processes and enabling oversight for study teams
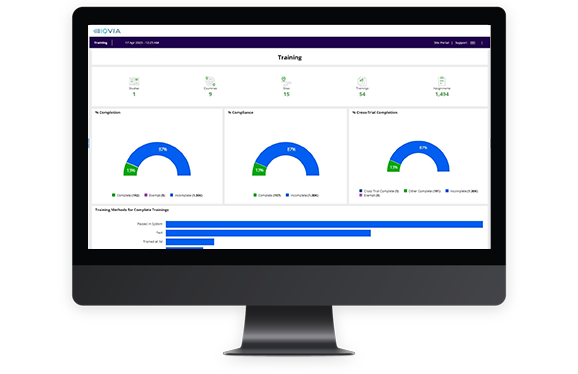
Accelerate site training and reduce the burden with automated assignments, cross-trial credits, and comprehensive oversight
Create and manage site submission bundles, including relevant study modifications and amendments, in one place
Eliminate paper/email-based manual processes
Ensure every study stakeholder knows what activities to complete by when
Get real-time oversight of start-up progress
Advance robust site adoption with minimal training
Gain efficiencies from vendor-agnostic integrations with CTMS, EDC, and eTMF
Speed site-activation timelines up to 50%
Learn ways to speed up site activation and provide a consistent site experience
Global Pharma Company Dramatically Improves Collaboration, Communication, and Compliance in Trials.
Reduce cycle time, speed site activation, and keep sites engaged with robust workflows and compliant eTMF filings
Communication, collaboration and transparency between sites and sponsors mean trials start up and close out faster.
Give your sites the courses and credits they need to succeed with the Learning Management module of the Investigator Site Portal.
Communicate SAEs, SUSARs, and other significant events to sites around the world with the Safety Notifications module of the IQVIA Investigator Site Portal.