















- Locations
- United States
- US Blogs
- Why Healthcare Organizations Can Benefit from New Sources of Real-World Data
Acquiring Data Blog Series: Part 1
The Data You Have Is Only A Beginning
Many not-for-profit healthcare organizations already manage valuable data about the patients or providers they serve.
Today however, medical specialty societies and patient advocacy organizations have an opportunity to do more. Shifting market conditions, such as the move to value-based care, integration of health systems and practice networks, and the adoption of innovative contracting models, to name just a few, are increasing both the supply of and demand for valuable data that can provide more comprehensive views of complex patient pathways (see figure 1) and help drive advancements in research and care.
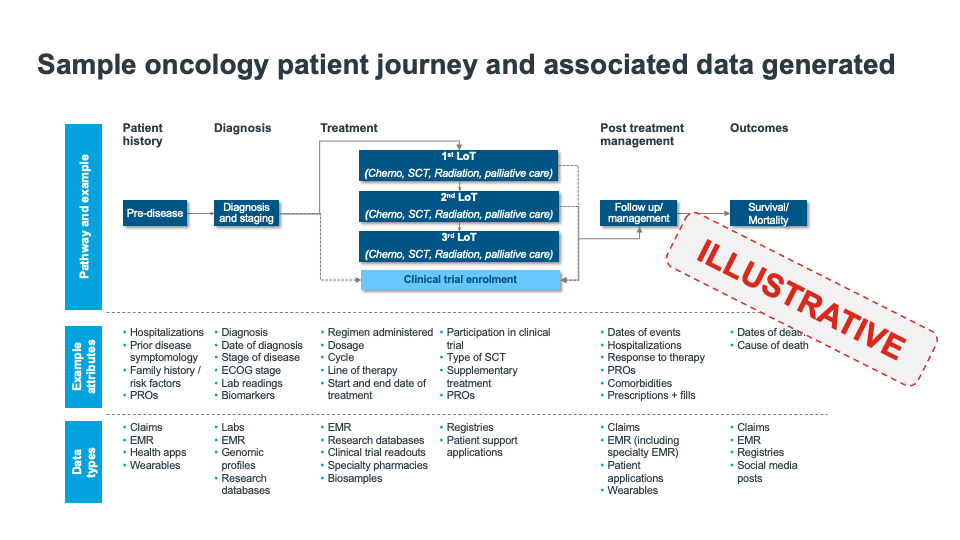
Figure 1: Sample oncology patient journey and associated data generated. Given the many touchpoints in modern patient journeys, it is challenging for a single source of data to provide an accurate, comprehensive view of a patient care pathway and associated quality, value, and outcomes.
Patient advocacy organizations and medical specialty societies are ideally positioned to take advantage of these market shifts and become the go-to data resource for their specialty area. Indeed, many life science companies are already looking to not-for-profits for these data and insights. By stepping into their new role as data stewards, patient advocacy organizations and medical specialty societies can bolster their convening powers, strengthen their collaborations with other health care stakeholders, and drive collective insights that will benefit patients and providers alike.
How New Data Sources Can Transform Your Organization
To take on this new role, not-for-profits need to seek out and acquire new sources of data beyond those they have traditionally collected. Acquiring these new data types can confer a host of benefits, including:
- Filling organizational data gaps to drive unique insights
- Creating and validating new value-based quality and outcome measures
- Providing a better understanding of patient journeys across the continuum of care
- Bolstering market offerings to improve your organization’s financial sustainability
- Fostering collaboration with other health care stakeholders
In addition to the broader benefits mentioned above, acquiring new data sources can also allow your organization to enable new use cases and offerings based on your data. These use cases can include:
- Real word research
- Trial recruitment / support
- Benefit design / value based contracting models
- QI / implementation science programs
- Patient satisfaction / preference research
- Utilization management / benchmarking
Which Types Of Data Should You Consider Acquiring?
The types of data you choose to acquire will vary based on your organization’s specific needs, as well as the current scientific and care paradigms of the therapeutic areas you function in. Most not-for-profit health organizations, however, will benefit from one or more of the following data types (please note, this list is illustrative of some of the most common data types used, it does not represent an exhaustive survey of all possible options):
- Electronic Health Record (EHR) data: EHR data sources that can provide longitudinal, clinical outcomes data on patient cohorts of interest are often the greatest value-add for not-for-profit orgs that already collect some level of patient or provider data
- Registries: Partnering with other registries can provide access to highly structured and high-quality datasets with variables of interest to your specific therapeutic areas, particularly for registries where data is directly inputted by physicians
- Claims data: Medical and pharmacy claims data sources are indispensable for health economics and outcomes research (HEOR) and health care utilization research. These data sources can include different providers and payer types based on the data source selected. Claims data are also a valuable source of information for organizations looking to demonstrate the burden of a particular disease to drive interest and funding.
- Patient-generated data: Patient-generated data is a diverse category encompassing everything from patient-reported-outcomes measures to data from health apps and wearables such as fitness trackers or portable glucose monitors. Patient-generated data is often particularly valuable for organizations interested in long-term follow up research, and for rare-disease focused organizations who are interested in developing and validating novel outcome measures.
- Biosamples / genomic data: While sourcing options for biologic samples and/or genomic data can often be more limited, such data is critical for organizations looking to drive deep scientific research in their therapeutic areas of interest, and to accelerate precision medicine and target discovery aims.

Figure 2: Pros and cons of common data types. Multiple types of data can often be used to strengthen organizational offerings and capabilities. Understanding the pros and cons of each type of data source can help you focus on the type(s) most appropriate for your situation.
Patient advocacy groups can often benefit from acquiring EHR data on their patients, particularly to enable them to link longitudinal clinical outcomes against the patient-facing registries that many patient organizations have maintained for years.
Medical specialty societies, on the other hand, may find less value in EHR data if they already obtain this data from providers in their quality registries. Instead, they may prefer to source claims or disease-specific registry data that could help them obtain a more complete view of the patient pathway, allowing them to more accurately capture and characterize the outcomes and risk factors that occur along each patient’s journey.
While these types of data can be valuable for many organizations (see Figure 2 for an overview of the pros and cons of each), please note that the ability of each type of data to address key unmet needs will vary by the dataset in question. This variability is generally driven by specific data metrics collected, the included patient population, the coverage of specific settings of care, and the overall geographic scope of the data source. Additionally, operational factors must also be considered in the validity of a data source, such as usage rights, quality, population coverage, and the ability to link to existing data assets.
Hopefully this overview has given you some inspiration regarding types of data that might help fill your unique organizational needs. In our next blog post, we’ll dive deeper into our proven process for identifying and assessing specific data sources and entering into productive data partnerships.
How You Can Get Started
For more information, such as organizations that have gone through this process, advice on identifying data sources relevant for your particular specialty area, or more details on data partnership and acquisition models, please contact Harvey Jenner or Jessica Preston at patientadvocacyteam@iqvia.com.
Read Part 2: Identify, Evaluate, and Integrate New Sources of Real-World Data
About the Authors
Harvey Jenner, Associate Principal, IQVIA Real World Networks
In his current role, Harvey leverages his scientific background and healthcare consulting experience to assist medical specialty societies and patient advocacy organizations to enhance their data-driven capabilities, provide sustainable registry value, develop research offerings, and navigate the complex data governance of multiple registries.
Harvey has been with IQVIA for seven years, managing large, global projects for pharmaceutical companies, providers, and other healthcare organizations. Prior roles include data and evidence strategy and implementation roles in Real World Analytics Solutions. He has a BSc in Biology from Imperial College London. Harvey is based in San Francisco, California.
Jessica Preston, Associate Consultant
Jessica joined the IQVIA Healthcare Solutions team in 2021 to support patient advocacy organizations and medical specialty societies in developing registry and data strategies that support their core mission while also contributing to organizational sustainability. Previously, Jessica worked as a research analyst supporting health systems and their partners with topics such as medical group medication management, specialty pharmacy, and health system service allocation.
Jessica received a Master’s in Public Health and a BS in Biomedical Engineering from the University of Virginia. She is currently based near Washington, D.C.




