















- Locations
- United States
- US Blogs
- Support Your Clinical and Economic Value Proposition with Real World Data - Part 2 in a series
The first post in this blog series addressed the use of real world data (RWD) at all stages of product development, and referenced IQVIA’s PharMetrics® Plus as a reliable data source. This blog will further examine how this comprehensive claims database effectively supports targeted pharmaceutical product initiatives, both independently as well as when it’s linked with other RWD sources.
IQVIA PharMetrics® Plus
Since 2006, PharMetrics Plus, IQVIA's health plan claims database, has enrolled over 190 million individuals, most of whom are commercial PPO and HMO patients. PharMetrics Plus is widely used by pharmaceutical and emerging biopharma companies across the full product development lifecycle.
This closed database of adjudicated medical and pharmacy claims has robust coverage for the under-65 population, and contains month-by-month information on patient enrollment, clearly indicating for each month whether a patient has pharmacy and medical benefits.
Patient enrollment criteria are essential to many studies. For example, a persistence study in which a patient’s use of a product is tracked over time, requires continuous enrollment during the study period to ensure that there is no gap in visibility into the patient’s medication use. Likewise, one can apply continuous enrollment criteria to identify true “first” diagnosis for newly diagnosed patients. By applying continuous enrollment criteria during the look-back period from the index event (first diagnosis of interest), we could check all the diagnoses the patient might have had during the study period. This is key to ensuring that the patient did not previously have this same diagnosis, and that the index diagnosis is, in fact, the first diagnosis.
There is a six-month lag on claims before they are included in this claims database due to the process of adjudication timeframe. This lag ensures that the data is complete and reflects true costs, as some claims can be processed for months after the actual date of service. The PharMetrics Plus database enables some pharmacy claims activity to be observed within 3-4 months for an early view.
In terms of cost, this data contains allowed amounts and paid amounts, which help to evaluate the true cost of care across settings. The database contains information on patient demographics including zip 3, patient diagnoses and procedures (international classification of diseases ICD‑9, ICD-10), current procedural terminology (CPT), and healthcare common procedure coding system (HCPCS) codes, medication use, hospitalization, and cost information (including allowed and paid amounts).
Another interesting data element available in this database is “confinement number.” This is a derived field that enables tracking the patient’s episodes during hospitalization. The data contain provider specialty information, and the majority of claims in more recent years include a national provider identifier (NPI).
Data is available in several formats, including native or observational medical outcomes partnership (OMOP) – common data model formats. Additionally, IQVIA offers a choice of delivery options, including via the Amazon Web Services (AWS) cloud or via IQVIA’s E360™ tool which automates basic data manipulation to facilitate quicker focus on mining and modeling.
The power of linking real world data
Linking real world data has been a growing trend in addressing complex research and business questions. Researchers are using novel approaches to gather different information from different databases to arrive at a more complete picture of a patient’s journey across care settings. Increasingly, it is being realized that relying on just one type of real world data might not be sufficient to meet complex research needs. In that light, PharMetrics Plus can be linked with other datasets, enabling patient data to be interconnected in a privacy-protected manner.
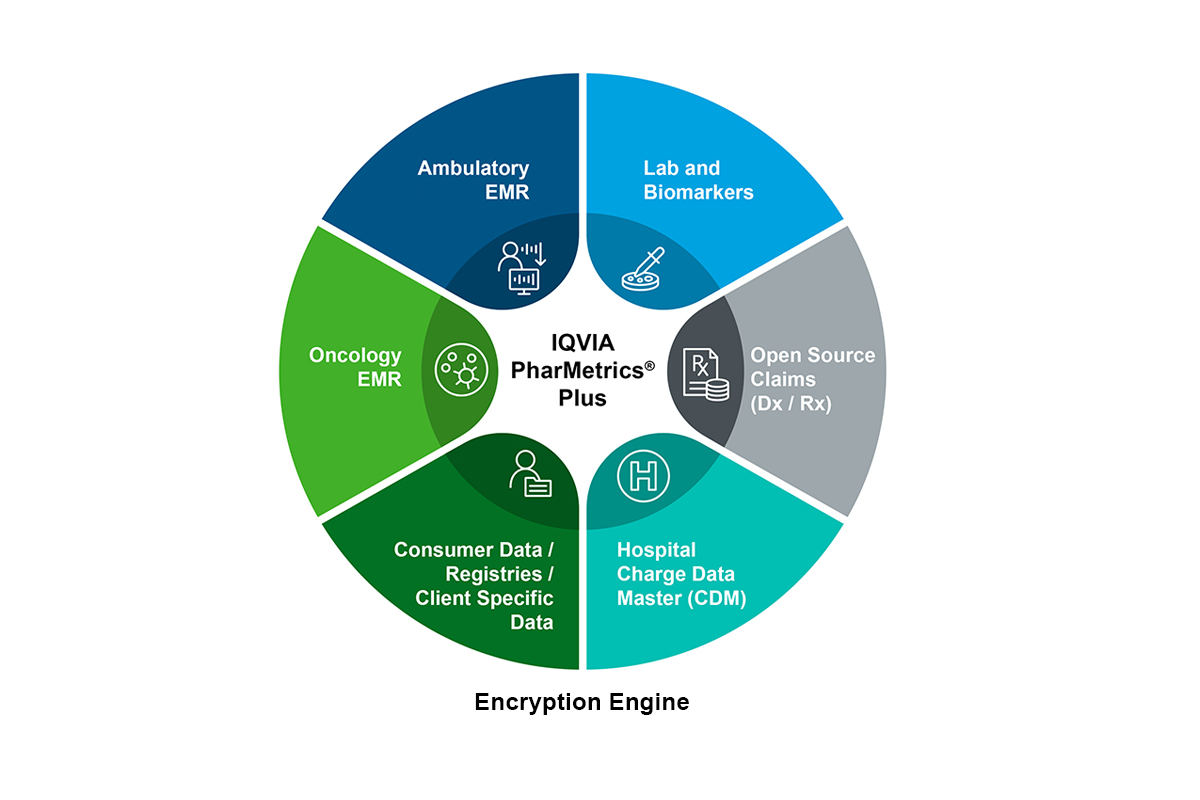
It can be linked to IQVIA and non-IQVIA data sources, including electronic medical records (EMR) data, along with data from labs, genomics, registries, patient-reported outcomes, wearables, and social media (FIGURE 1). For example, when defining outcomes based on BMI or other clinical metrics, one would link PharMetrics Plus to the ambulatory EMR (AEMR) database, identify patients using diagnosis codes in the claims database, and link to obtain clinical metrics.
Figure 1: Linking to other data offers the unique ability to uncover deeper and more specific insights.
A PharMetrics Plus use case
In a study of oncology patients, an analyst might link PharMetrics Plus to IQVIA's oncology EMR to obtain tumor growth and staging information. If a research team is interested in rare diseases not yet identified with ICD-10 codes, AEMR data can be mined to obtain text strings, signs and symptoms, and systemized nomenclature of medicine (SNOMED) codes to identify appropriate patient cohorts, and then link to PharMetrics Plus to obtain comorbidities, resource utilization, and other outcomes for those patients. To incorporate cash payments not captured in the insurance claims, one can link to IQVIA’s open source pharmacy claims database to augment PharMetrics Plus.
Learn more about the functionality and effectiveness of a patient claims database through more detailed PharMetrics Plus use cases featured in the final blog in this series.





