















- Locations
- United States
- US Blogs
- Support Your Clinical and Economic Value Proposition with Real World Data - Part 1 in a series
New databases and advanced technology facilitate the generation of meaningful evidence for healthcare stakeholders, from healthcare cost effectiveness, to clinical safety and efficacy.
Use of real world data (RWD) in drug development and research continues to expand, particularly in these areas:
- Increased usage of RWD for guidelines and policies for regulators and payers
- Continued usage of claims data as the foundation for real world research
- Augmentation of claims data with richer clinical elements to support key therapeutic areas
- Application of artificial intelligence/machine learning (AI/ML) to do more and more with big data
FIGURE 1 illustrates the many potential sources of real world data (RWD). Health insurance claims data is perhaps one of the most widely used sources of RWD by the pharmaceutical industry due to its availability, representativeness, and measurement of medically attended events and cost. Other RWD sources include those with clinical richness, such as lab and biomarker data or electronic medical records (EMRs). Technology advances are encouraging the collection of more data directly from patients through wearables and apps to collect patient-reported symptoms.
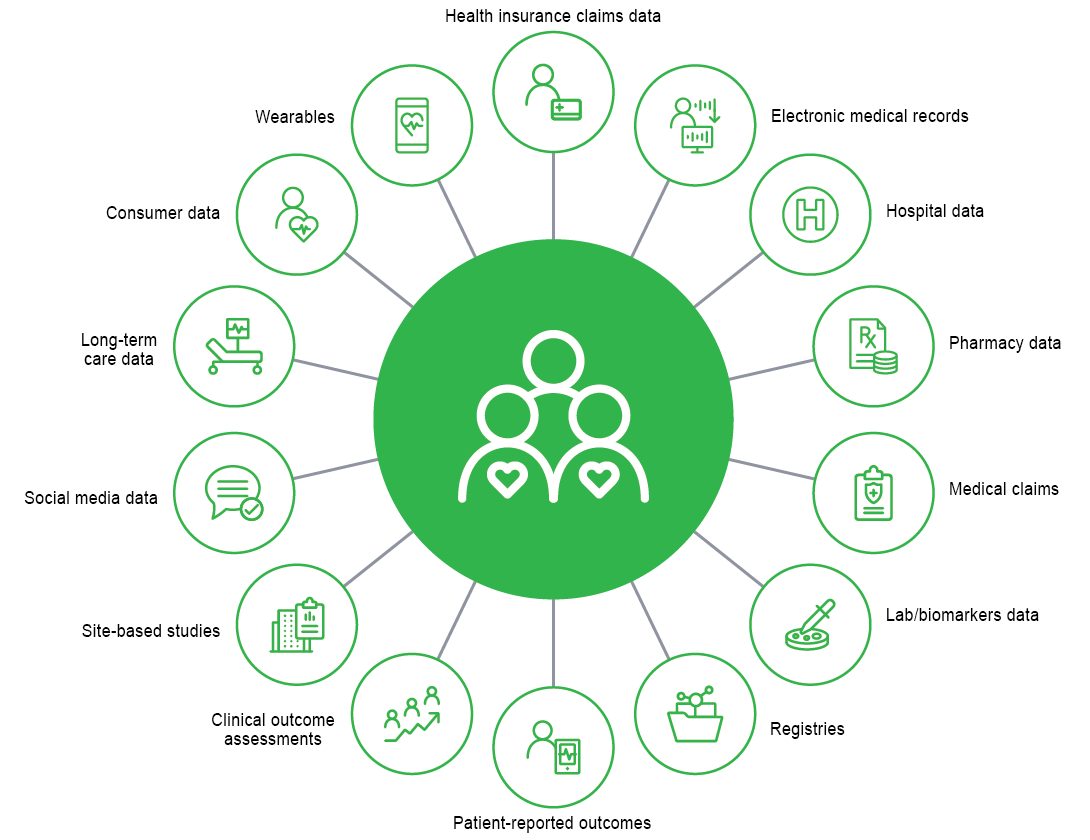
For years, healthcare claims data has been used in the later stages of the drug development lifecycle, during and post-launch, to target key physicians, evaluate cost effectiveness, estimate new-to-brand market share, and understand the patient journey. More recently, healthcare claims data has increasingly been used earlier in the drug development process to help researchers test trial designs and understand potential screen-out rates. Clinical trial recruiters can locate sites with higher populations of specific rare diseases. And currently, RWD is being used in regulatory submissions to support evidence needs for rare diseases in single-arm trials. RWD can be used throughout the product lifecycle, from early phase through post-launch.
- Early-phase applications for RWD include market opportunity assessment, forecasting, competitor analysis, external comparator/synthetic control studies, and incidence and prevalence analysis. Regarding the latter, to evaluate the prevalence of diabetes among women between the ages of 20 and 40, for instance, one could query a database such as IQVIA’s PharMetrics® Plus to see how many patients have those characteristics among women in the 20-40 age group in the database with enrollment details. The enrolled women in the database essentially provide a built-in denominator, and the women with diabetes make a built-in numerator to calculate prevalence in that specific population.
- In late phase, RWD analyses might include cost of treatment for standard of care and burden of disease. This application relies on comprehensive cost information across different settings of care. A database of RWD like PharMetrics Plus can provide a window into the entire burden of disease—including whether the patient has been hospitalized, the cost of drugs that have been administered/dispensed, the cost of doctors’ visits, and more.
- Post-launch RWD applications include studies of comparative effectiveness, cost analyses, persistence and adherence, label extension, and patient journey. RWD can also be leveraged for patient journey analysis.
The next post in this series, highlights how PharMetrics Plus, IQVIA’s health plan claims database and just one of the organization’s many data sources, can be an important part of accessing and harnessing real world data.





