Strengthen your portfolio by making more informed decisions and prepare for the impact of new research discoveries.






















- Blogs
- Advancement of Treatment with Biologics in CNS Diseases
Use of biologics in the central nervous system (CNS) field is growing rapidly, with sales of $19 billion in 2018 increasing to a forecast $49 billion in 2032 according to IQVIA’s Forecast Link. A number of diseases are behind this growth, including migraine, myasthenia gravis and muscular dystrophy as seen in the graph below.
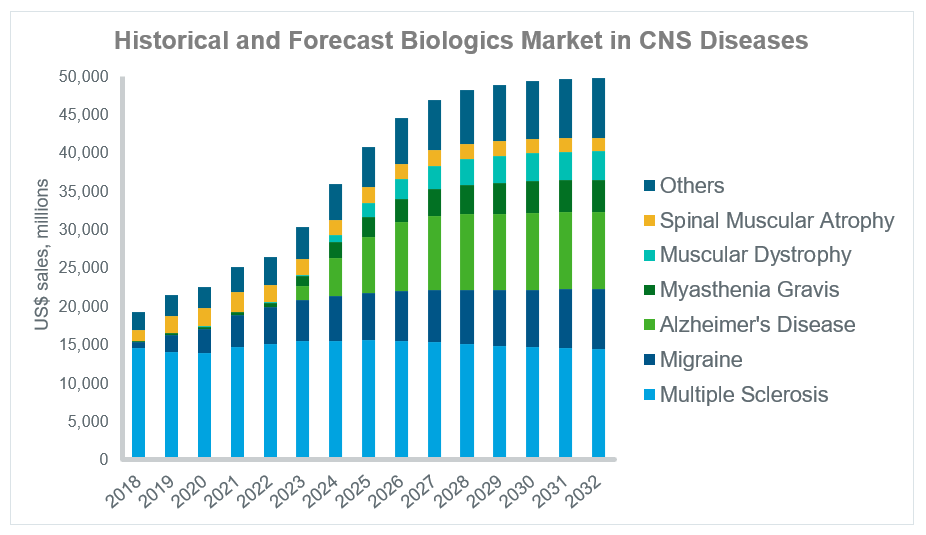
Source: IQVIA Forecast Link, July 2023
In comparison, growth of non-biologics in the CNS field, which includes small molecules, has increased from $105 billion in 2018 to $140 billion in 2032. Non-biologics contribute the vast majority of the market share, but new biologics are providing growth.
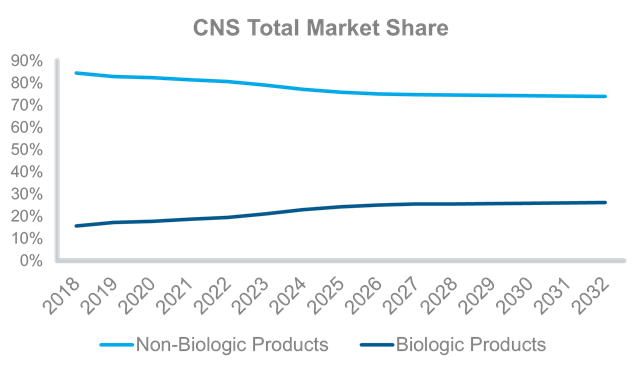
Source: IQVIA Forecast Link, July 2023
Migraine has seen significant growth in the use of biologics in the last 5 years, with four Calcitonin gene-related peptide (CGRP)-antagonists currently approved for treatment. These biologics offer the benefit of preventing migraine episodes and reducing the number of headaches patients experience per month. Prior to this, therapies for migraine prevention were not migraine specific and were associated with low adherence due to poor tolerability and insufficient efficacy1.
Biologics have also transformed multiple sclerosis treatment options with the introduction of multiple CD20-positive B-cell depleting monoclonal antibodies. An issue that biologics development in CNS diseases face is the ability of drugs to cross the blood brain barrier because monoclonal antibodies are large molecules. Therefore, large doses are given to ensure a sufficient dose reaches the brain and this also increases the risk of serious side effects2. Disease progression in relapsing-remitting MS may be due to lack of penetration of the blood brain barrier by these monoclonal antibodies3.
Whilst some CNS diseases have seen major progression in recent years and show strong sales forecasts, others such as Alzheimer’s disease lag behind. With an aging population, there is significant need for a therapeutic that can slow, reverse or stop development of the disease, which is devastating for patients and families and the burden on healthcare systems and communities is substantial. The unmet need and large patient population means there are potentially extremely high profits to be made but achieving this is proving difficult. Drug developers have faced failure after failure with pipeline monoclonal antibodies. However, in recent years two biologics have received approval and although the path has not been smooth, these approvals open the gateway to future growth and forecasted sales are positive in the disease.
Drug development in Alzheimer’s disease has been a frustratingly slow process, but patients, families and experts are cautiously optimistic that as the understanding of both the disease and how to gain regulatory approval grows, so will treatment options.
For more information on brand and country specific forecasts in any disease, please contact IQVIA and request a demonstration of Forecast Link, an online tool containing 5 years historical and 10 years of forecast data in 73 countries, for 10,000 drugs and over 600 diseases. Forecast Link provides unrivalled breadth of coverage and is updated quarterly. To find out more, please visit Forecast Link: Disease.
References
- https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01211-5
- https://pubmed.ncbi.nlm.nih.gov/33756057/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5605202/
- https://pubmed.ncbi.nlm.nih.gov/35516416/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7697739/





