





















- Blogs
- Brief Overview of Supply Chain Management Systems and Supply Chain Resilience
Introduction
The term Supply Chain Management (SCM) was introduced by Keith Oliver in 1982 and was defined as the process of planning, implementing, and controlling the operations of a supply chain to ensure the smooth flow of a product from a raw material to a finished product followed by its delivery to a customer.[1] Since then, the term has evolved to include each process starting from procurement to after-sales service.
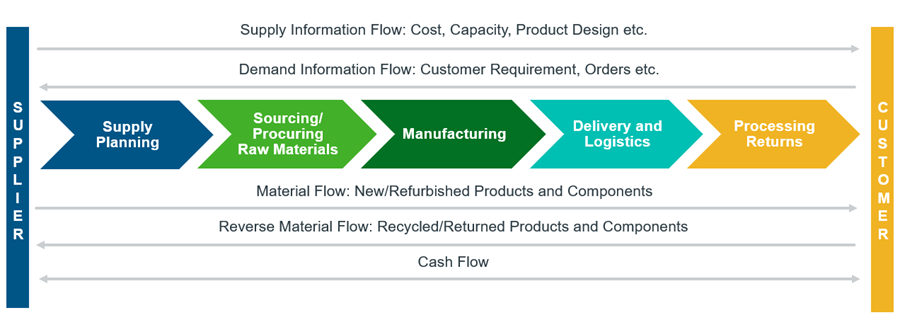
A traditional SCM system has five components (Figure 1):
- Supply Planning
This involves developing and establishing a supply chain strategy based on customer demand and company inventory for a product. Most companies make use of certain analytical tools to match supply with demand and develop a course of action. This stage also involves organizing a company’s warehouse and/or manufacturing facility. - Sourcing/Procuring Raw Materials
Identifying suppliers that can provide the raw materials and services required to manufacture the product. Establishing a system to monitor and manage supplier relationships and productivity. This includes processes like ordering, receiving, managing inventory, and authorizing supplier payments. - Manufacturing
Transforming the raw material into the final product. This stage includes synthesis, testing and packaging of final product. - Delivery and Logistics
Coordinating with customers in scheduling and dispatching deliveries, invoicing customers, and receiving payments. - Processing Returns
Establishing a system to take back unwanted or defective products from customers. The firm may also return excess or expired raw materials back to the vendors or suppliers.
Supply Chain Resilience
Disruptions in Supply Chain: Supply chain disruption can be defined as a breakdown in the flow of an SCM system. Supply chain disruptions can be caused by natural disasters, man-made accidents (explosions, fires), geopolitical situations (tariff hikes, civil unrest), internal company matters (product recalls, machine breakdown, financial issues) and security and cyber theft. These are just a few common examples of disruption events, but the list is not exhaustive. Some of these events can be predicted and are easier to plan for, like colder winters or the monsoon season, but some of these events, like natural disasters or conflicts, are at times unexpected and therefore hard to mitigate as well.
It has been identified that, since 2009, globally, more than 50% of companies suffer a supply chain disruption annually.[2] To cut costs in the competitive business environment, there is a growing preference for outsourcing and offshoring. Since multiple third parties are involved, it reduces the direct visibility and the control a company has over their supply chain. As a result, supply chains are becoming increasingly fragmented, complex, and interdependent. So, a disruption at any level creates a snowball effect and leads to a huge revenue loss for a company.[2]
One unfortunate situation that we see in the pharmaceutical industry because of supply chain disruption is drug shortages. According to a 2019 drug shortage report by the United States Food and Drug Administration (FDA), 62% of the 163 drugs that went into shortage between 2013-2017 were preceded by supply chain disruptions associated with manufacturing or product quality problems. As per the FDA, one of the root causes of drug shortage over the past few years was logistical and regulatory challenges after the supply chain disruption. To recover from a supply chain disruption, companies that have outsourced production to other countries would need to begin production locally or at some other offshore location that has not been affected by the said event, for which they would need regulatory approvals and cost-effective processes. Other countries that wish to enter the US market also need regulatory approval, which they may not get immediately in case of a disruption event. Moreover, approvals are required not only from the FDA but also from regulatory bodies in other countries wherever manufacturing operations are to be commenced. Models for upgrading manufacturing facilities developed by International Society for Pharmaceutical Engineering estimated that it can take up to 7 years to obtain approvals from global regulatory authorities because of varying timelines and requirements across different regulatory bodies. Industry studies for small-molecule generic drugs in the United States have estimated that FDA approval procedures for bringing a drug into market can easily take up to 3-5 years.[3] This incapability to recover from a supply chain disruption over time leads to drug shortages in the market. It also leads to a rise in the drug price as supply drops below demand. Furthermore, the generic drug market usually has very few suppliers and is greatly impacted in case of a drug shortage. If one company ceases production owing to any internal or external issue, the other companies would need to substantially increase their output in a short period of time to meet the demand, which is quite challenging.[3]
Owing to the above-mentioned factors, it has become highly imperative for a company to build a resilient supply chain. A resilient supply chain is defined as one having the capability to respond to and recover from disruptions by maintaining continuity of operations and having control over the supply chain flow. As part of a resilient supply chain, a company needs to have certain contingency plans in place. Any kind of disruption calls for the following actions:
- Detecting the impact and degree of disruption
- Designing a recovery method to mitigate/tackle the disruption
- Deploying the recovery method
As part of their recovery strategy, companies need to be prepared with
- Alternative suppliers for sourcing and buffer stock to meet customer demands. It is also a good practice to maintain multiple suppliers to ensure that customer demands are met in times of shortage.
- Alternative transportation modes
- Additional production capacity for continuing production
Additionally, having good coordination and visibility among all elements of a supply chain is integral for an effective recovery.[2] Also, having flexible regulatory frameworks in place allows parties to mitigate any local/offshore production challenges that they may face in case of a disruption event.
The pipeline incident at BASF showcases a good example of a resilient supply chain. In 2016, a BASF facility in Germany faced a pipeline explosion which destroyed a terminal that was being used for the supply of raw materials. To mitigate this, BASF shifted its logistics from ships and pipelines to trucks and trains. BASF showed good coordination with its customers and kept them informed of the current availability of the products and was successful in minimizing the impact on customer deliveries. In this instance, BASF managed its supply chain in a way that promoted flexibility and considered safety and risk prevention measures.[2]
Digitalization of SCM: Currently, the supply chain landscape is undergoing rapid digitalization. Companies like BASF and Avaya have successfully adapted and implemented digital supply chains, which integrate internal systems and data with external information.[4,5] By making use of new technologies to collect and monitor data, a digital supply chain can help you make predictions, bring in price transparency and recommend actions in realtime. It also helps the companies to understand the supply chain dynamics and react quickly to market shifts. Endto- end digitalization of a supply chain helps with performance management and optimization of even the most complex supply chain. By minimizing the manual planning component, this approach enables the planning process to become smoother and more responsive towards real-time situations, hence making a supply chain more resilient.
Also, rather than opting for an end-to-end solution, a lot of companies opt for taking advantage of digital platforms available in the market for some of the steps. Digital sourcing platforms are a good example of this. A digital sourcing or procurement platform helps in analyzing and making the right choice while selecting a supplier. They also provide the customer with an opportunity to directly connect with a supplier and negotiate a deal.
How IQVIA Chemical Intelligence can help?
Finding the right supplier is a crucial step in the sourcing component of a supply chain. There are a lot of options out there when looking to source raw material, so weeding out and choosing the right supplier becomes a challenging task. This is where sourcing platforms are valuable. They bring together information from diverse sources in a uniform way and help analyse and make an informed decision. They are an ideal place to find multiple suppliers for a raw material.
IQVIA Chemical Intelligence is a sourcing platform that connects chemical and pharmaceutical buyers and sellers with valuable sourcing data. The platform lists more than 489,000 chemicals linked to more than 13,600 suppliers. The Directory of Producers module helps identify suppliers of chemical raw materials and active pharmaceutical ingredients and enables a customer to selectively seek out suppliers that are International Organization for Standardization/Good Manufacturing Practice certified and/or have submitted a Drug Master File to the FDA or hold Certificates of Suitability from the European Directorate for the Quality of Medicines & HealthCare. The Page 4 of 4 © 2023. All rights reserved. IQVIA® is a registered trademark of IQVIA Inc. in the United States, the European Union, and various other countries. Finished Forms module helps identify suppliers of pharmaceutical dosage forms. This module also helps to find potential buyers of active pharmaceutical ingredients and raw materials. The Contact Manufacturing module offers more than 200 other pharma and non-pharma services and helps in choosing the right company for outsourcing of production. The Synthesis Pathways module offers alternate routes of synthesis for a chemical and helps to find potential leads for selling chemical intermediates.
Conclusion
SCM is an integral part of most businesses. Having an efficient SCM system in place helps boost a company’s profit by reducing purchasing and production costs. It also helps companies to predict and identify potential problems and have a contingency plan in place. A well-developed supply chain infrastructure also boosts the country’s economy by enabling exchange of products between businesses and consumers quickly and efficiently at low costs. SCM also plays a crucial part in disaster relief operations and other medical emergencies. Also, the advent of digitalization in SCM has created extensive opportunities in terms of reducing human error and analyzing and controlling the supply chain flow in real-time.
References
- Lauren Xiaoyuan Lu, Jayashankar M. Swaminathan, 2015, ‘Supply Chain Management’, International Encyclopedia of Social and Behavioral Sciences, 2nd edition, Vol 23.
- K. Katsaliaki, P. Galetsi, S. Kumar, 2021, ‘Supply chain disruptions and resilience: a major review and future research agenda’, Annals of Operations Research
- https://www.fda.gov/media/131130/download
- https://www.basf.com/global/en/who-we-are/digitalization.html
- https://www.avaya.com/en/about-avaya/newsroom/news-180206/
IQVIA™ Chemical Intelligence is a leading information provider to purchasers, sellers and researchers in the Pharmaceutical and Chemical industries worldwide. To find out more, please click here.





