





















- Blogs
- Digital Biomarkers
The convergence of analytics technology, wearable devices, and machine learning has led to a new form of data for clinical research: digital biomarkers.
A digital biomarker is a quantifiable indicator of a patient’s physiological and/or behavioral state captured via any number of patient-wearable connected devices, including but not limited to, wearable (i.e. blood pressure monitors, actigraphy devices or multi-sensor patches) and implantable (i.e. pacemakers, continuous glucose monitors), technologies.
The evolution of these devices, and the ability to remotely capture this data is providing researchers and physicians with a windfall of novel near and real- time insights. That’s good news for Alzheimer’s Disease (AD) research where the impact of this data will be profound:
24/7 data capture
Wearable and implantable devices can continuously capture data with little or no required patient interaction, providing researchers with hundreds or thousands of data points per day related to the patient’s condition. Additionally, many of these devices now have real-time, or near real time, communication capabilities, providing researchers and caregivers richer data sets, trending information, and details regarding specific events. This information can be used to assess safety and efficacy endpoints as well as to development of predictive algorithms along with tracking physiological endpoints, such as blood pressure and heart rate, and research teams can track behavioral trends related sleep patterns, activity levels, diet, and day-to-day habits. In combination with cognitive surveys and other validated instruments conducted via digital devices, this unbiased and ongoing flow of data (data flow) will provide detailed and accurate profile of a patients’ physical and mental status over time.
In studies focused on prodromal or pre-symptomatic patients, such data, in combination with pharmacogenomics, can deliver new insights into the early progress of this disease. It will also be crucial for identifying which digital biomarkers – or combinations of biomarkers (i.e. sleep patterns, WASO and nocturnal blood pressure dipping) -- are the strongest indicators of early AD risk.
These discoveries could speed the clinical recruiting process and reduce the high rate of screen failures for future AD trials.
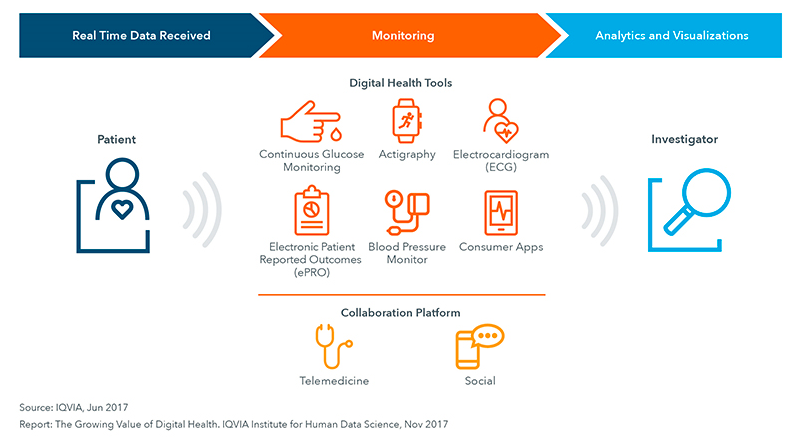
Figure 1: Digital Health Use in Clinical Trials for Patient Monitoring
Less disruptive
In any clinical study, researchers need to balance the need for patient data versus patient burden. Frequent site visits and demands for additional tests can cause participants to drop out of trials, or to fail to comply with the treatment.
Capturing digital biomarker data via connected devices may reduce this risk. When researchers gather information remotely and automatically, it lessens the need for patient site visits while delivering a more consistent quantity and quality of data. These devices can also add value for patients and caregivers who want to track their status, and/or receive alerts or reminders to take a medicine or complete tasks.
In AD clinical trials, as well as long-term observational studies, this is huge benefit. Prodromal patients in particular are difficult to find, so retention of every patient is vital. For studies tracking patients in the later stages of dementia, reducing site visits lessens the burden for patients and caregivers, allowing researchers to capture data in the homecare setting, where patients are less likely to become agitated, aggressive, or more confused.
Reduced overhead
Site monitoring is a significant cost in clinical development. Clinical Research Associates (CRAs) spend hundreds of hours visiting sites to monitor and validate data capture and transcription because so much information is collected from patients during face-to-face visits. Use of validated digital tools to automatically and accurately collect endpoint data and transfer it to the clinical database will decrease site management requirements while increasing data collected and improving data accuracy.
Going forward: We must make better use of data
Regulators are increasingly supportive of using data captured from wearables and other connected devices in clinical trials because they see the value these endpoints bring to our understanding of disease progression.
The challenges now include determining which technologies and devices available now can deliver useful data today, how best to use this data to support safety and efficacy endpoints and how to combine these data streams into data sets that can be used to develop more useful algorithms to guide research and treatment tomorrow. Every incremental advance in the speed and quantity of data we capture via connected digital health devices creates new opportunities for the AD research community to advance our understanding on this disease.





