















- Locations
- United States
- US Blogs
- Establishing an Integrated Evidence Plan for Medical Affairs and Beyond
Over the life of a biopharmaceutical product, there are many situations when data from randomized controlled trials (RCTs) may not be sufficient to address stakeholder questions of value, safety, and effectiveness. In a healthcare system that is working to improve value, innovation, and healthcare quality, we know that collecting new data through RCTs can be impractical and prohibitively expensive.
For biopharmaceutical companies, generating the right real world evidence (RWE) to augment RCTs is a strategic imperative for drug development and commercialization.
Moving beyond this realization to knowing how to best prioritize and generate the necessary evidence is a great challenge. Internally within biopharmaceutical companies, there may be competition for resources, uncertainty about the types of studies needed, or ambiguity about the trade-offs or level of evidence required for decision making. Furthermore, these dynamics may change by local market and indication over time, and they may be complicated by combination therapies and precision medicine approaches that involve more than one product or process for patient screening, treatment, and reimbursement.
What is an integrated evidence plan?
Integrated evidence plans (IEP) chart a collection of evidence requirements, endpoints, data sources, study concepts, and target audiences using a common structure and language that can be used across the sponsor organization. IEPs are intended to be shared and debated across functions, prioritized, and modified as study concepts are finalized, feasibility assessments are completed, and as the market context changes. The IEP catalogs all existing and planned evidence, and it seeks to identify unmet evidence requirements, dependencies, and common themes across different categories of evidence.
With all the unknowns, changing competitive landscape, and competing priorities, how do you optimize an evidence generation plan? How do you anticipate needs in a dynamic market to advance a cohesive brand strategy? A well-coordinated cross-functional effort is needed to collect the best fact base and define the appropriate depth of research. It’s important to create a common atmosphere of participation and ownership across functional teams, and to develop a layered approach that defines key problems and a roadmap of potential solutions that can be refined and executed against over time.
How to best approach integrated evidence planning
IEPs can be approached in different ways depending on stakeholder needs and market context. For example, IEPs can be developed at the franchise or product level and updated at key points in the product lifecycle. IEPs are especially useful when planning to enter new markets, when products are newly acquired, or when competitor market events introduce changes or uncertainty in the commercial landscape.
Importantly, IEPs may also incorporate communication requirements and evidence dissemination plans. Generally, a busy period for IEP creation and updating is about 12-24 months prior to expected regulatory approval. The research and dissemination projects that emanate from the IEP can sometimes require that length of time to plan, execute, and read out. But where does a team begin when technical knowledge and information about a product and future commercial markets are spread about the organization?
Creating an integrated evidence generation plan
When developing an IEP plan, it makes sense to first define the key business and research questions to be answered. Key questions can be created and revised throughout the planning process, but starting with a set of focused questions is an important step to gain alignment across an organization. Perspectives across stakeholders can differ, and collecting a variety of viewpoints through expert interviews and advisory boards can offer a starting point for topic categorization and prioritization.
As a next step, the available evidence should be cataloged and assessed against the Target Product Profile (TPP) and brand plan. Sometimes key evidence already exists, and it is important to create an organized fact base from which to conduct a gap analysis to reveal unmet evidence requirements.
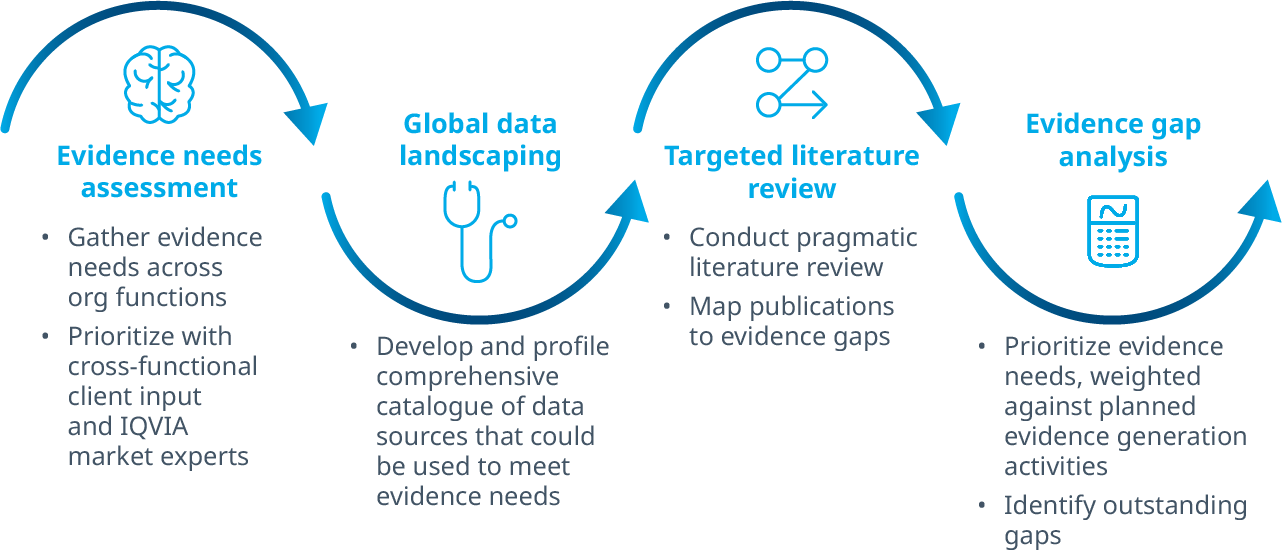
When gaps in the evidence requirements are identified, the next step involves planning to address the gaps. At this stage, teams should generally keep their planning efforts at a high level to keep progressing with momentum while leaving room for refinements as the IEP comes into focus.
Data sources may be landscaped, and study concepts developed, but teams should avoid too much detailed resource planning at this stage as the IEP is still intended to invite edits and changes to prioritization across functions. Efforts should be made, however, to continuously map and prioritize evidence requirements against the TPP and brand plan to ensure targeted efficiency and examination of the risks of potential negative outcomes.
When a comprehensive collection of prioritized evidence requirements has been developed, the IEP should be validated with the core team and a selection of external thought leaders who can thoughtfully weigh the regulatory and/or market access objectives of the IEP.
The result is an IEP aligned across key functions and markets that identifies what evidence will be collected, for whom, and in what sequence. Based on this plan, sponsor organizations can identify and generate the required evidence in the most efficient way, while addressing the most important priorities in the context of the product lifecycle and market dynamics.
Keep the plan current and dynamic
Evidence needs will change, as the market evolves with the launch of competing products, patent expirations, and the development of a deeper understanding of a disease’s underlying biology. At the same time, the data itself will undoubtedly change over time as more information is collected. IQVIA recommends that companies revisit IEPs periodically, or upon learning of an external market event that will change the framework against which decisions are made.
The benefits of an integrated data strategy
As outlined in the graphic below, there are five key benefits that will result from a properly constructed IEP.

The need for greater efficiency in product development and the growing power of key stakeholders to shape the success of a drug demand that companies adopt a strategic and agile approach to generating and disseminating evidence.
Read on to understand how and when to develop a medical communications plan to disseminate the evidence generated from real world data sources.

RWE Academy on Call
Let IQVIA’s Real World Evidence Academy on Call help you enhance your RWE knowledge base, enable process implementation, and further RWE communications within the healthcare ecosystem. Learn more in this fact sheet.




