Maximize your healthcare investments, with evidence.
After hitting a five-year low in 2022, biopharma M&A activity has seen a dramatic rebound in the first six months of 2023, with aggregate deal values up by 133% vs. the first half of 2022 to reach $82Bn. These deals include specific criteria, involving a biopharma target, acquisition of a majority stake in the target entity, and total consideration equal to or exceeding $1Bn, including contingent payments linked to reaching certain milestones. Product and division acquisitions and licensing deals or similar are not included.
Highlights include the re-emergence of mega-deals, valued at $25Bn or greater, with the pending $43Bn Pfizer-Seagen acquisition, coming hot on the heels of the pending $28Bn Amgen-Horizon deal announced at the close of 2022, while the majority of big pharma players opted for bolt-ons, e.g., the $10.2Bn Merck-Prometheus deal, the $6Bn Takeda-Nimbus acquisition, the $3.2Bn Novartis-Chinook deal or Lilly’s $1.9Bn deal to acquire DICE Therapeutics. This trend in renewed deal momentum bodes well for the full-year 2023 outlook.
Fundamentals in support of dealmaking remain strong
Deal-making continues to be strategically important for the biopharmaceutical industry, as big pharma players seek to replenish their pipelines, access novel capabilities or sharpen strategic focus, while financially hard-pressed emerging biopharma companies (EBPs) are eager to explore exit options.
Strong drivers of supply and demand create favourable fundamentals for dealmaking, which highlights the interdependencies across the biopharma innovation ecosystem. Specifically,
Demand drivers:- The biopharmaceutical industry is facing a growth gap as it is approaching its next patent cliff. For example, in the critical U.S. market alone $210 – $250Bn of revenue will be newly exposed to loss of exclusivity (LoE) between 2023 and the end of the decade. Among top 15 pharma, 30% of U.S. revenue will be exposed, putting ~$120Bn of collective revenue at risk.
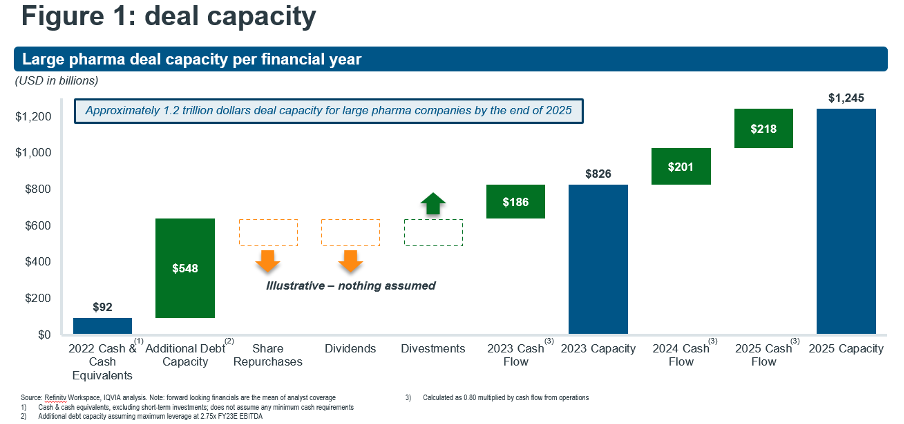
- At the same time, big pharma has amassed a formidable war chest for deployment in dealmaking, equating to $0.8Tn deal capacity among the top 15 companies by 2023 and growing to $1.2Tn through 2025 [Figure 1: deal capacity].
- EBPs are at the forefront of bio-medical innovation and accounted for two thirds of the industry’s clinical-stage development pipeline in 2022, which saw an all-time high for the number of pipeline assets. As such, EBPs become prime targets for the external sourcing of innovation by larger players.
-
EBPs are facing a tough funding environment, with total run rate biotech funding levels in the U.S. down by nearly 40% in H1 2023 vs. peak funding in H1 2021.
- An essentially still closed IPO route restricts access to public markets. As at June 2023, the Nasdaq Biotechnology Index recorded a mere 7 biotech IPOs compared to 21 and 104 for the full year of 2022 and 2021, respectively.
- Private funding (VC and PIPES) in the U.S. has been more resilient, with $12.1B raised year to date. However, while VCs have sufficient dry powder, they reserve capital to support their existing portfolio companies as late-stage funding via the IPO route has dried up, leaving less capital for new investments.
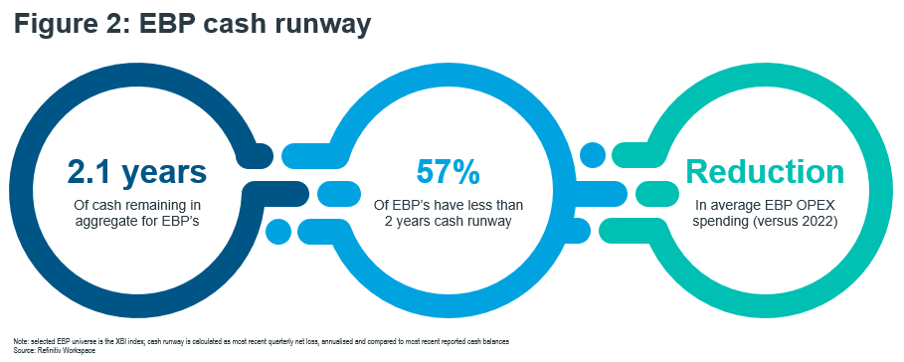
- The recent failure of Silicon Valley Bank, which served EBPs as a key customer segment, has added to market anxiety and exposed the precarious cash position of many of its clients. Our analysis estimates that 57% of the SMID-cap EBP universe have a cash runway of less than 2 years at current spending rates [Figure 2: EBP cash runway].
- The market correction starting in 2021 and playing out in 2022 brought EBP valuations down to earth. As at June 2023, the XBI index is down by 49% from its pandemic-fuelled, frothy high in February 2021. We are seeing a return to ‘fair value’ and focus on quality anchored in compelling and mature clinical data. Consequently, early-stage companies with preclinical assets or with vague technology platform promises have been hit particularly hard, with valuations down by 70% or more, while de-risked, late-stage assets have shielded companies from the full brunt of the market shake-out.
The confluence of those supply and demand drivers has removed key barriers which hampered deal activity in 2022 and results in a change from a seller’s to a buyer’s market, with both sides highly motivated to complete transactions.
Uncertainties remainDespite strong fundamentals and a rebound in deal activity in the first half of 2023, key uncertainties are weighing on dealmaking sentiment:
- Recent legislation in two major markets, the Inflation Reduction Act (IRA) in the U.S. and the draft revision of the EU pharmaceutical legislation (draft EU legislation), introduces uncertainty and has the potential to disrupt the biopharma business model, with far-reaching implications that may act as both barriers and catalysts for dealmaking.
- The IRA's effect on the space is multifaceted, encompassing both potential drawbacks that could compress takeout valuations and overlooked advantages that may positively influence deal flow. One the one hand, acquirers may apply a risk premium if small molecule-based drugs end up with shorter patent protection, as well as taking increased rigor in the due diligence process. Indeed, this stricter regulatory climate may require additional efforts from EBPs, even already during phases I and II, to substantiate differentiation of the benefits of their innovation. Conversely, shorter asset lifecycles may further increase demand for deals to replenish revenue which is facing even greater/earlier exposure to LoEs, legal challenges recently filed by some manufacturers against the IRA further exacerbate uncertainty around the shape of the future operating environment.
- The draft EU legislation is also expected to impact asset lifecycles by expediting the availability of generics and biosimilars, or by using exclusivity to incentivise innovators, e.g., rewards for ensuring availability of drugs in all EU member states, or for running comparative trials. However, it is important to note that its immediate impact is unlikely, as adjustments are expected to occur during the consultation stage.
- The Federal Trade Commission (FTC) has adopted a more activist stance and increased its scrutiny of transactions, e.g., challenging Amgen’s proposed $28Bn acquisition of Horizon Therapeutics on anti-competitive grounds, which may have a chilling effect on overall dealmaking sentiment and especially on companies pursuing larger transactions. Recently, the FTC proposed updated regulations that would impose additional information requirements on deals, and if implemented, could considerably lengthen the filing process for companies.
- The broader macro-economic and geopolitical outlook remains challenging, e.g., a high inflation/high interest rate environment persisting for longer, or deglobalisation creating new barriers to cross-border sourcing of bio-medical innovation and its commercialisation.
In the second part of our dealmaking blog, we will discuss the outlook for 2023, including a full year projection of deal activity, implications of key uncertainties for expected deal focus and where to find value.
Key data and information sources
IQVIA Pharma Deals; IQVIA Protection Link; IQVIA Forecast Link; Mergermarket; Refinitiv Workspace; IQVIA Institute report: Global Trends in R&D 2023; company financial reports; company press releases and deal announcements; IQVIA EMEA Thought Leadership desk research and analysis

Exploring strategic alternatives? Reach out to us!
Related solutions
See how we partner with organizations across the healthcare ecosystem, from emerging biotechnology and large pharmaceutical, to medical technology, consumer health, and more, to drive human health forward.

























