















- Locations
- United States
- US Blogs
- Using Life Science Funding Opportunities To Achieve Financial Sustainability For Patient Registries
Creating and maintaining a high-quality registry to collect and manage information about your patient community can support your mission in multiple ways. A research-ready patient registry can help you to:
- Aggregate important but hard to find data for a disease community relating to the care, outcomes, and experiences of patients and their support networks (for example, patient insights and testimonies that can support patient-centered drug development)
- Share important information about symptoms, disease progression, and treatments with your patient community
- Attract researchers toward studying your disease area(s)
- Create opportunities for collaborations with life sciences companies who can both create needed treatments and products for your patients, and provide your organization with funding to support other mission-critical activities
- Provide real-time support and insights back to patients to support their care journey
Patient registries can thus fill a key niche in the healthcare ecosystem, and provide a critical resource to support research, speed the development of new therapies, and collect and amplify otherwise unknown patient voices and perspectives.
Building a research-ready patient registry is expensive
Building an agile, next-generation registry that can incorporate multiple data types is increasingly attractive to patient advocacy organizations who want to strengthen their ability to support research and treatment development. However, these registries are often prohibitively expensive, especially for patient advocacy organizations with smaller budgets. For a medium to large, rare disease advocacy organization, we estimate that costs for registry technology along can span from $0.5 million to $2 million for initial setup, with ongoing yearly maintenance costs of between $300 thousand – $1 million. (These costs are highly dependent on the patient and treatment dynamics in the specific therapy area, proposed registry size, the data collection mechanism used and the use cases that will be utilized of the back of the registry.) Depending on their staffing model, organizations may also face additional costs related to hiring and training staff to manage the use of their registry and associated programs.
Although a next-generation patient registry can be expensive to set up, once it is built it can enable you to provide value to multiple stakeholders, address multiple organizational goals, and will have the ability to evolve and adapt as your needs change and as the overall landscape of your therapy area advances.
Closing the funding gap and promoting financial sustainability
A research-ready registry provides value to stakeholders across the board, including patients, researchers, clinicians, and industry. However, due to their unique positioning, patient advocacy organizations are usually the ones who take on the upfront investment in these valuable resources because of their existing, trust-based relationships with patients and caregivers.
Thankfully, patient advocacy organizations have an opportunity to leverage the expected value of their registries to help offset the costs of the registry build and support ongoing maintenance.
In this article, we’ll cover ways organizations are working with life science partners to support both the upfront build and ongoing maintenance of their registries.
Funding opportunities from life sciences before you build your patient registry
In recent years, life sciences companies and patient advocacy organizations have begun working more closely together on joint initiatives and research studies. This increased collaboration can be beneficial to both parties; however, these relationships are often complicated, and can be perceived negatively if not set up and overseen correctly.
Industry can be engaged at many stages of the registry lifecycle, but a growing trend is the formation of pre-competitive consortiums, where patient organizations seeking to build a registry pursue early investment from a small number of Life Science supporters who are active within the therapy area.
Founding members provide seed funding in exchange for the ability to shape registry development, in addition to access unique data and/or services once available. Access to data can be restricted as appropriate (for example, only providing de-identified data extracts, or analytical reports), based on the intended uses of the registry and the comfort level of your stakeholder community (including patients, executive leadership, scientific advisors, and research community). Effective data governance is critical to successfully managing these partnerships.
For this approach to succeed, there must be sufficient demand for data in a particular disease or therapy area from life sciences organizations. Without this demand, new registry projects are unlikely to attract much interest or pre-competitive funding from life science organization. There will generally be high demand for data where there is both a reasonable pipeline of incoming treatment options for a therapy area, as well as limited existing data assets.
Life sciences funding opportunities for ongoing registry maintenance and program support
Once you have a registry in place there are opportunities for you to generate revenue using your data and/or patient network associated with the registry.
One way that patient advocacy organizations with an established registry can generate revenue is by delivering a range of patient engagement, data offerings and research services to support life sciences companies in developing new treatments and getting them to patients. In addition to generating funding to offset registry and other program costs, such services can also accelerate a patient advocacy organization’s mission by supporting care and outcomes improvements through improved clinical trial awareness, as well as broader data use to generate insights regarding burden of illness, disease progression, and healthcare utilization, including equity and access.
It’s important to note that not every organization is equally suited to standing up service offerings for life sciences organizations. Some organizations will be limited by the depth and quality of their data, their analytical capabilities and/or the consent models they have in place. Others will be primarily limited by organizational appetite for these kinds of services, particularly if there is concern about real or perceived conflicts of interest that could damage an organization’s reputation, violate data governance policies, go against organizational mission and beliefs, and/or damage patient relationships.
However, when engaged in thoughtfully and with the proper safeguards in place, industry-centric offerings can offer significant, lasting sources of revenue to help you fund the maintenance or enhancement of your existing registry and programs.
Potential offerings generally depend on the maturity of your data and capabilities
For organizations that do decide to offer services to life sciences organizations and other partners, there is considerable variation in the depth and range of services offered. Additionally, these offerings and services can take multiple years to build up, with different offerings enabled over time (see Figure 1) as the organization’s capabilities develop and the value of the registry and patient network increases.
Case Study: Rare Disease Advocacy Offering and Funding Roadmap
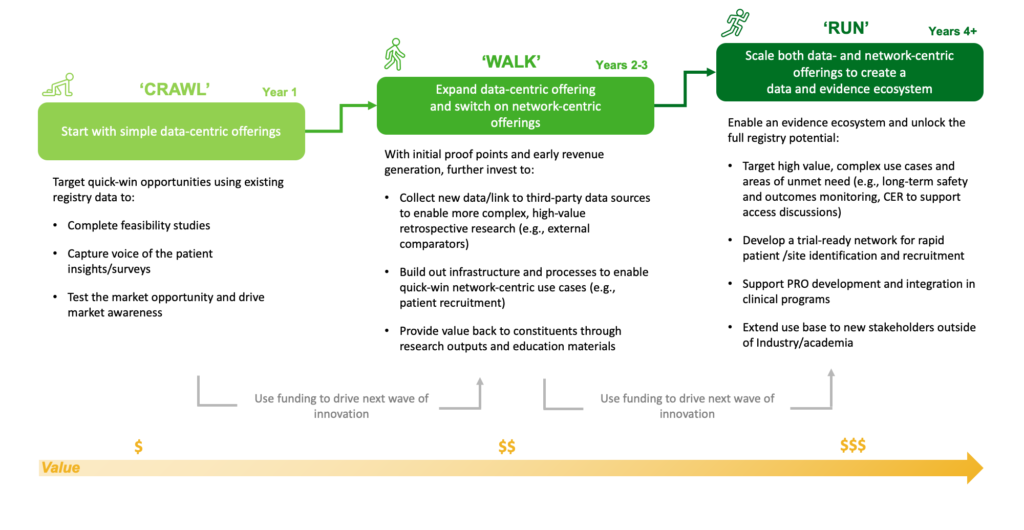
Figure 1: Roadmap for the development and expansion of a rare disease registry.
How you can get started
For more information about other organizations that are pursing sustainable registry funding options, as well as to discuss the best options for funding a registry build or enhancement in your disease area, please contact Harvey Jenner or Jessica Preston at patientadvocacyteam@iqvia.com.
For more information on specific revenue-generating offerings that can promote organizational sustainability, see our sustainability whitepaper
About the Authors
Harvey Jenner, Principal, IQVIA Healthcare Solutions
In his current role, Harvey leverages his scientific background and healthcare consulting experience to assist medical specialty societies and patient advocacy organizations to enhance their data-driven capabilities, provide sustainable registry value, develop research offerings, and navigate the complex data governance of multiple registries.
Harvey has been with IQVIA for 7 years, managing large, global projects for pharmaceutical companies, providers, and other healthcare organizations. Prior roles include data and evidence strategy and implementation roles in Real World Analytics Solutions. He has a BSc in Biology from Imperial College London. Harvey is based in San Francisco, California.
Jessica Preston, MPH, Associate Consultant, IQVIA Healthcare Solutions
Jessica joined the IQVIA Healthcare Solutions team in 2021 to support patient advocacy organizations and medical specialty societies in developing registry and data strategies that support their core mission while also contributing to organizational sustainability. Previously, Jessica worked as a research analyst supporting health systems and their partners with topics such as medical group medication management, specialty pharmacy, and health system service allocation.
Jessica received a Master’s in Public Health and a BS in Biomedical Engineering from the University of Virginia. She is currently based near Washington, D.C.




