















- Locations
- United States
- US Blogs
- Secondary First A Better Approach to RWE
The use of secondary database studies is the foundation of real-world evidence (RWE). These studies provide valuable insights that can be gleaned quickly and easily from existing data, also called real-world data (RWD). The benefit of these models is leading many industry experts to take a ‘secondary data first’ approach to their evidence strategy.
The premise is simple: Primary data actively collected are difficult, slow, and expensive to obtain. Secondary data already exist, as they were captured for their primary purpose in healthcare. They can be reused for another, secondary purpose to gain insights. When facing a scientific or business question first consider whether you can find an answer in the existing data -- internally, among partners, or in a network.
What are primary, what are secondary data
RWD contain clinical patient data obtained from claims and electronic health records (EHR), but also those from primary sources, such as study and registry data. Non-medical data from journal articles and social media feeds are also considered RWD.
How to use RWD
These data can be queried, or studied, for purposes across the entire pharmaceutical value chain: drug research, clinical development, and commercialization. For example, they can be used while drugs are still in R&D to:
- Understand the current standard of care
- Identify unmet medical needs or existing treatment obstacles
- Trace disease progression, diagnosis, and treatment trends
- Assess the safety and efficacy of existing treatment interventions
- Support protocol design and feasibility to optimize studies from primary data
- Identify the best sites and most appropriate patients for studies from primary data
Or, for your products after approval, to
- Conduct safety surveillance and signal detection
- Demonstrate cost of illness and Healthcare Resource Utilization (HCRU)
- Collect evidence for negotiating optimal pricing, reimbursement, and coverage
- Forecast and track brand performance
- Support targeted campaigns, inform messaging, and hone market strategy
The use of RWD for evidence is a rapidly growing trend, and one that is increasingly accepted by regulators, HTAs, and payers as proof of evidence.
Secondary data challenges
Before using secondary RWD as part of the clinical or commercial strategy, consider what data sets you will want to use, how you gain access to it, and how you will link them together if they are from disparate sources. Sometimes, this is not straightforward.
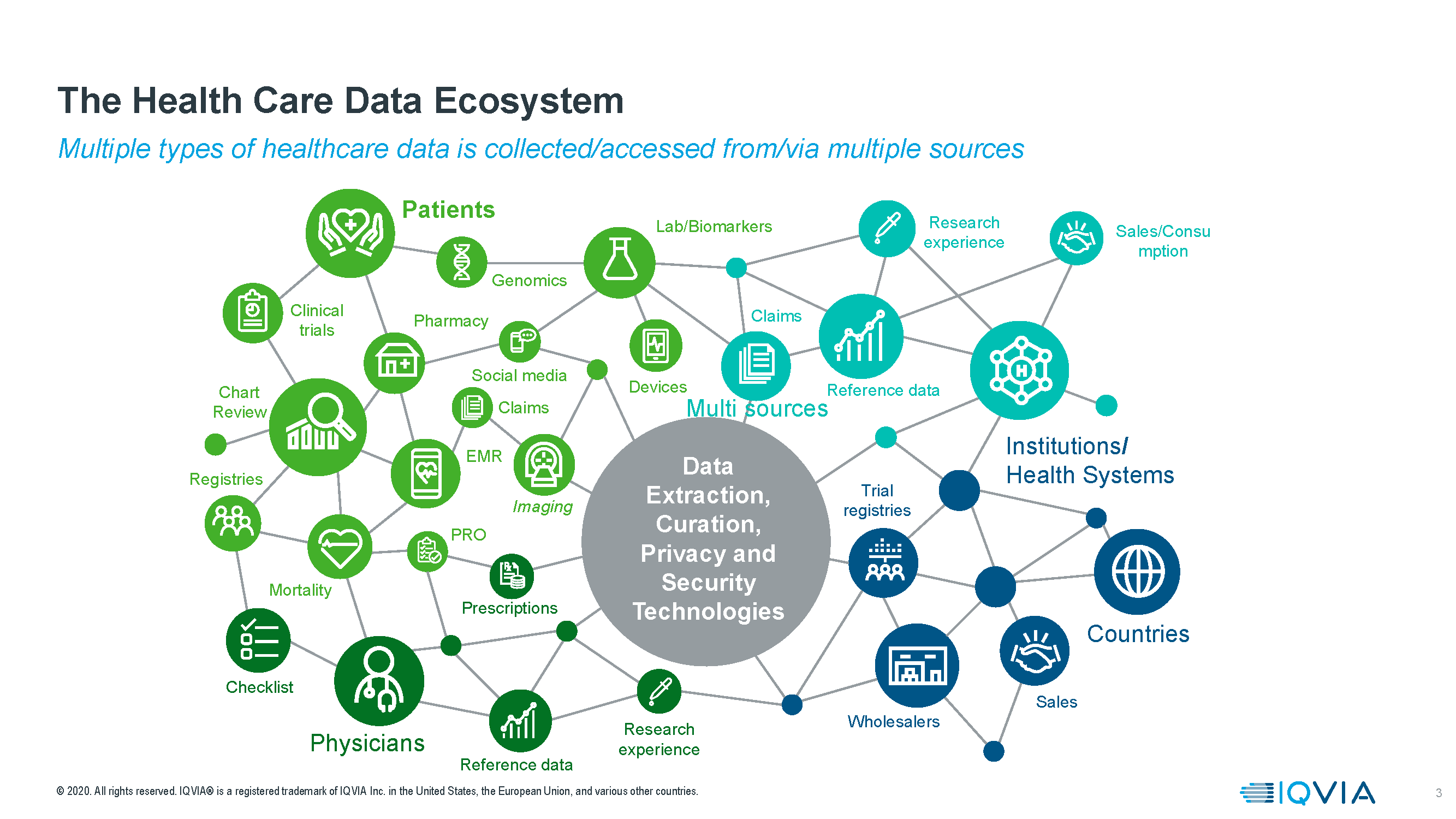
Secondary data are generated for their primary purpose. The consequence of that provenance is that they follow their own languages, structure, platforms and data formats. Access or dissemination, even for legitimate research purposes, is tightly limited by their primary owners and governed by rules and regulations for personal data protection. It is also not guaranteed that any RWD are fit for your purpose. Some have unique strengths and weaknesses, which often only reveal themselves in the analysis process.
For example, claims data, which are generated to manage reimbursement requests, very reliably capture robust information about the service and the underlying disease. But they typically lack such details as symptoms, lab tests, imaging results, or progress notes. So, depending on your question, you may or not may find what you need in the data. Conversely, EHRs contain a lot more of such details, but are recorded by individual physicians without the same completeness or rigor of standardization. In addition, the data may or may not be structured. A lot of it is hidden in narrative notes not amenable for large-scale computerized analysis. Natural language processing can help, but not supplant the formalized data capture into a structured format.
Non-clinical data present an entirely different set of challenges. Articles, conference session notes, social media posts, and any other narrative reports are much more complicated to analyze because of its complete lack of context or structure.
Finally, the development of commercially available secondary data is a rapidly growing industry still undergoing a steep maturation process to arrive at generally accepted levels of quality, reliability, transparency, and technical foundation.
What’s your strategy?
To achieve the most benefit, you may want to develop a comprehensive secondary data strategy that is defined early in the process: Which data sources, which technology to clean, code, de-identify, and structure the data, and how to analyze it.
When you are undertaking such an effective secondary data strategy, you want to work with experienced partners with knowledge about data access and analysis. That will save you time and cost.
IQVIA is the largest global healthcare data vendor in the world and has conducted numerous secondary data studies. Therefore, we can address these challenges, either with our own data, the evaluation of other secondary data, or the application of our analytics expertise.
To learn more, click here to listen to the IQVIA webinar, IQVIA Real World Data and OHDSI Network, or contact us.





