Navigating the complex global patent landscape.
New White Paper Available: EU Revision of the Pharmaceutical Legislation. To request access, email thoughtleadershipgroup@iqvia.com
On 26 April 2023, the European Commission proposed their long-awaited revisions to the EU’s pharmaceutical legislation aimed at making medicines more available, accessible and affordable. We analysed five of the more contentious proposals, applying IQVIA’s unique data and insights to explore their impact on life science companies, payers and, ultimately, evaluate the extent at which the Commission’s proposals may shake up European healthcare.
The five areas are:
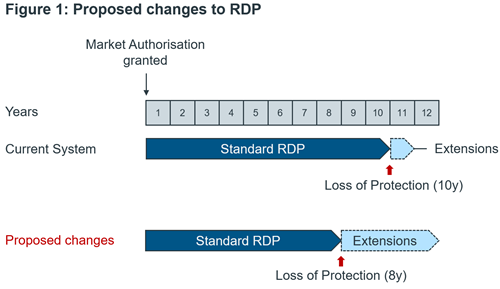
- Reliance on Regulatory Data Protection (RDP). These changes, see Figure 1, need to be looked at in combination with the wider EU Pharmaceutical Strategy for a full assessment. Companies will be impacted differently, depending on their portfolio. We provide an analysis on what this means for different stakeholders.
- Changes to orphan medicines. There may be a smaller consequence on how orphan medicines are brought to market. We go into details in the report.
- Access across all EU member states within two (or three) years. IQVIA believes that launching within two years is not possible under the current decentralised system.
- Transferrable anti-microbial resistance (AMR) vouchers. We discuss the voucher’s potential as an incentive and propose a hybrid purchasing framework instead.
- Incentives for the repurposing of medicines. Finally, repurposed medicines are in the spotlight. But will these changes be enough to allow them to gain a price premium?
How can you prepare?
Although this proposal will likely undergo change and not be implemented until the second half of this decade, it is important to engage in this conversation early and calculate its impact on your organisation.
These are the essential steps IQVIA recommends to ensure you are ready:
- Understand the impact the proposal changes will have on your portfolio and future acquisition strategy
- Pressure test launching across all EU member states, evaluating supply needs and building launch capabilities
- Find partners able to support launching in EU member states
- Calculate what value the AMR vouchers could have for your organisation
- Evaluate repurposing opportunities to capitalise on new RDP incentives
Want to find out more?
To request the full white paper with deeper analyses, or if you would like IQVIA to profile how this proposal may impact you, please reach out to your IQVIA representative or directly to thoughtleadershipgroup@iqvia.com
Related solutions
Generics is about competition
See how we partner with organizations across the healthcare ecosystem, from emerging biotechnology and large pharmaceutical, to medical technology, consumer health, and more, to drive human health forward.
Analyze industry leading sales and medical data in a standardized and comparable way, including cross-country analysis of performance and additional insights into global and regional markets.
Get an up to date view of key performance indicators globally, regionally, or locally.
Be proactive about growing your brand using the latest in data, analytics, and domain expertise.
Take advantage of the latest tools, techniques, and deep healthcare expertise to create scalable resources, precision insights, and actionable ideas.

























