















- Locations
- United States
- US Blogs
- Evidence Dissemination: How to Make the Most of Your Real World Evidence for Medical Affairs and Beyond
In an earlier post, we described how life sciences companies can create an integrated evidence generation plan to map out what real world evidence (RWE) they should be seeking, by audience, geography, and timeframe. Here, we go one step further to explain how to approach the communication of RWE strategically, rather than transactionally.
The mantra, “Begin with the end in mind,” is often heard in clinical development, meaning that achieving the desired impact on clinical practice and patient outcomes should be baked into development plans for the greatest impact.
In the same way, an understanding of how real world evidence (RWE) will be used – and to what effect – should be infused into RWE generation plans. Too often, medical communications professionals are handed the results of a study at its completion, and while publication of study results in a peer review paper is valuable, it is often the minimum that can be achieved to maximize ROI.
Viewing real world evidence strategy through a communications lens
We recommend that medical communication professionals be an integral part of the early discussions around, and preparations for, developing a business case for any RWE initiative. Their involvement and functional support at this stage will help with the following:
- Warranting that the resulting value story will be as strong as possible. Professional communicators will approach the data generation plan from the perspective of how the data will be received by each set of stakeholders. What decisions will those stakeholders be making, and how will the study data influence those decisions? By viewing research plans from this perspective, communicators can often make recommendations for modifying the data elements to be collected in some way that will be more productive in the end. “If only they had thought to include X or to cut the data differently…” is a familiar refrain among communications professionals, and such regrets can easily be avoided by seeking their input from the outset.
- Supporting the business case to senior management. The business case for a research undertaking will be more compelling if it describes the intended impact and how that will be achieved. When a communication plan grows out of the evidence generation plan, it becomes clear that study results will not collect dust on a shelf but will be used to move the needle in decision making. Communications experts can help craft the positioning for a study to ensure that its aims, methods, and value are clearly understood, data are well anticipated, and the study value maximized.
- Preparing internal teams to make use of the results. It is important that the broader internal team supporting a product know what research is being undertaken and for what purpose, how it fits into the overall brand strategy, and when various results will be published. Since research can take several years to complete, internal team members should be kept apprised of the status. With consistent messaging and frequent communications to the team, busy medical affairs leaders in local markets can anticipate what is coming and will be prepared to act on newly published material. It is even possible to provide internal teams with digital visualization tools so that they can intuitively gain a deeper understanding of the data as it becomes available.
- Allowing for a forward-thinking communication plan. When medical communicators know in advance what is being researched and how it will support the scientific narrative, they can develop a strategic dissemination plan that pulls through the messaging that is appropriate for each audience, considering how and when it will have the most impact. A carefully crafted plan might, for example, maximize exposure by parsing out the findings, releasing one cut of the data in one journal in year one and another cut in a different journal in year two. Or, it might recommend that three data points be combined to generate one high-impact paper rather than three less important ones.
- Tailoring communications to the stakeholder. Plans to report study results should be customized for each stakeholder audience, considering their understanding of the clinical subject matter and their preferred sources of information. A given outlet, such as a peer-reviewed journal, is not always the most effective way to reach audiences beyond healthcare professionals; other avenues may well be more appropriate for reporting study results to payers and patient groups.
The value of an end-to-end approach
Beyond seeking early input from communication professionals into the initial RWE generation plan, it can be advantageous to turn to the same partner to both generate the data, analyze it, and communicate it.
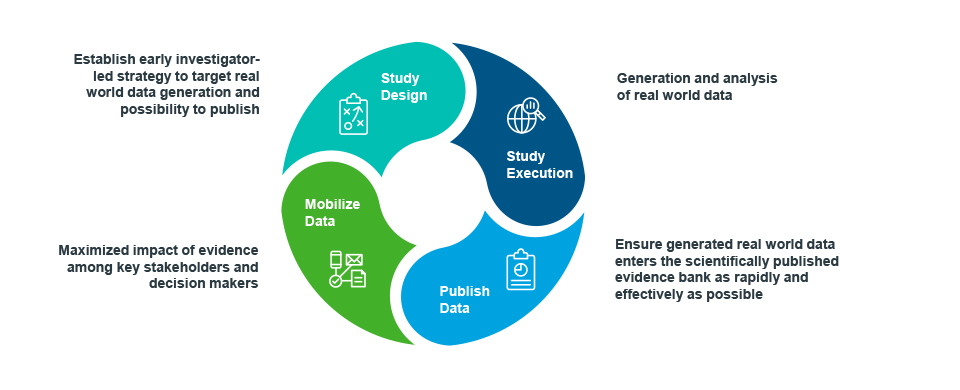
There are obvious synergies and efficiencies to be gained in reducing the number of handoffs and in tapping the expertise of data scientists in readying the data for publication. There’s alignment on the objectives from start to finish, the insights incorporated are understood first-hand, and the timing of analyses and submission dates are easily coordinated.
To learn more about how IQVIA’s approach to medical communications planning can help your organization maximize its use of real world evidence, get in touch today.





