CRISPR-based therapeutics have entered the clinics and 2023/24 could bring the first regulatory approval. This is the current chapter of a story that started in the late 1980s. CRISPR was known to be able to cut DNA, but the exact mechanisms remained elusive. Almost 30 years later, a team of scientists led by George Church, Feng Zhang, Jennifer Doudna and Emmanuelle Charpentier discovered that CRISPR could be used as a tool to precisely edit mammalian cells. Doudna and Charpentier were later awarded the Nobel Prize in Chemistry for their pivotal contributions. This eureka moment led to the founding of an increasing number of start-up companies that want to leverage CRISPR in healthcare, diagnostics or in agriculture.
What makes CRISPR so appealing is not only its precision but also high efficiency, versatility and – more importantly – its simplicity compared to other gene-editing technologies. In brief, CRISPR can be programmed to target and disrupt the function of a gene or, if provided with a template, can add desired sequences to the genetic code (Figure 1 upper panel).
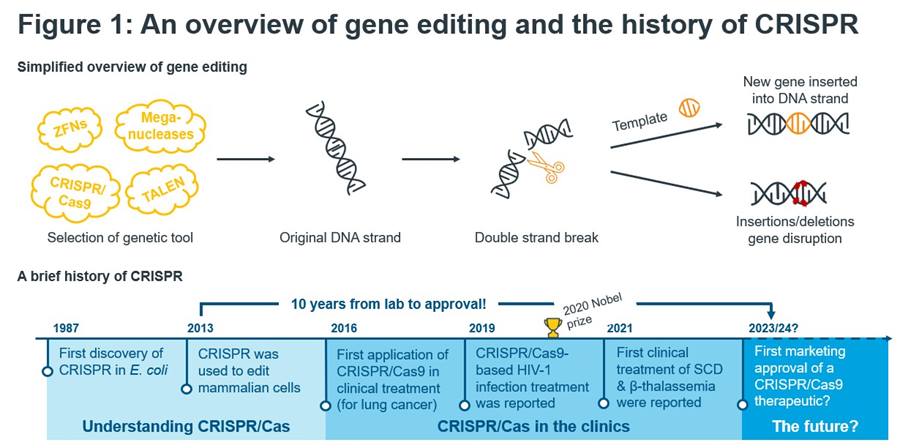
Figure 1: An overview of gene editing and the history of CRISPR.
Sources: Nature, National Institute of Health, Nature, Science Direct; IQVIA EMEA Thought Leadership.
The incredible CRISPR story does not end here. Only 10 years after lab experiments in petri dishes, Vertex and CRISPR therapeutic’s Exa-cel filed for regulatory approval in the US, Europe, and the UK. In this second instalment of our series of regular articles on developments in the space of cell, gene, and RNA therapeutics, we want to acknowledge these rapidly evolving field and give an outlook on the gene-editing innovative landscape as well as a perspective for the entire field.
Pipeline
CRISPR/Cas9 is the driving factor behind clinical research into gene-editing therapeutics with the potential to be curative for genetic diseases. As Figure 2a shows, whilst there are multiple technologies in the clinical pipeline, more than three-quarters of products are based on CRISPR/Cas9. When considering the broader CRISPR platform, over 80% of all products are based on CRISPR. The most advanced asset is Exa-cel for the treatment of patients suffering from sickle cell anaemia (SCA) and is an ex-vivo approach where a patient’s own cells are extracted, gene-edited to produce high levels of foetal haemoglobin and thus remove the transfusion requirement1. Strikingly, in data presented last year, the treatment was almost 100% effective after three years2. Exa-cel is racing towards approval, whereas the overall gene editing pipeline is in early clinical development. Two-thirds of gene-editing products are at the pre-clinical stage.
In contrast to the first approved gene therapies, Exa-cel would be a therapeutic against a highly prevalent condition. SCA is a widespread disease, affecting approximately 300,000 new-borns each year, which is expected to rise to 400,000 cases by 20503.
The use of gene-editing therapies is also being investigated in a wide array of therapy areas, as illustrated in Figure 2b. Over a quarter of the gene-editing pipeline is concerned with blood disorders (including SCA), with the next largest area being cancers. Importantly, many of the products in this area are looking to produce CAR-T therapies using CRISPR/Cas9 to edit T-cells, rather than directly using gene-editing on cancer cells. CNS disorders also represent a significant portion of the pipeline, with products looking to target diseases such as Huntington’s and even Alzheimer’s.
Exa-cel is not alone in targeting a very prevalent condition. Over two-thirds of the pipeline is targeting non-rare diseases, across all therapy areas mentioned above. Gene-editing therapies are being investigated to target everything from HIV infections to non-small cell lung cancer, compared to the earlier approved gene therapies which were focused on very rare conditions.
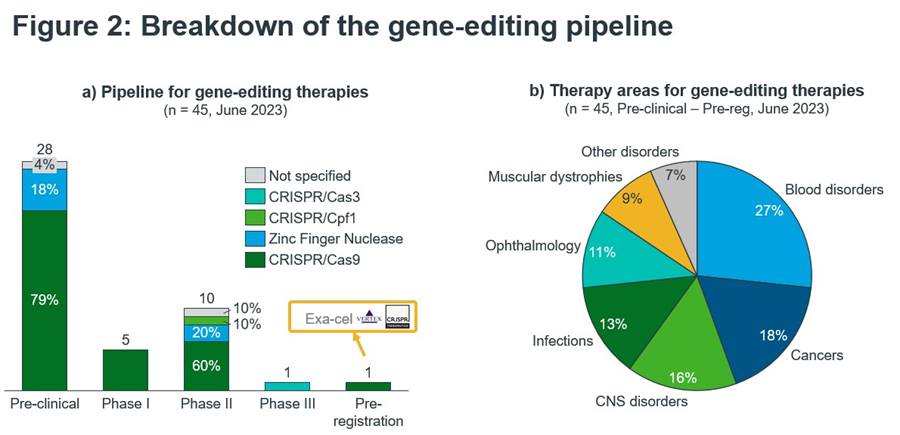
Figure 2: Breakdown of the gene-editing pipeline.
Sources: IQVIA Thought Leadership, IQVIA Pipeline Intelligence June 2023, search criteria: Descriptor = “CRISPR-Cas”, “CRISPR-Cas9”, “CRISPR-Cpf1”, Zinc finger nuclease”, “base editing”.
Looking at earlier-stage research alongside the clinical pipe, CRISPR-based therapies are being investigated across even more indications. Diabetes is one of the areas of interest. Vertex and CRISPR Therapeutics have recently continued their partnership, with CRISPR Therapeutics receiving $100mn from Vertex to help develop gene-edited cell therapies for Type I Diabetes4. There are also investigations into the use of gene-editing to help treat anxiety, with lab tests in mice showing how CRISPR/Cas9 can be used to target the HTR2A gene and reduce expression, though of course this is at very early stages5.
The future of gene editing might not rely solely on CRISPR/Cas9 but will use other subtypes like Cpf1 or Cas3 instead. Moreover, base editing – a newer gene editing technology that uses components of the CRISPR system – has hit the clinics in 20226. Verve therapeutics utilises an in vivo approach to precisely knock out the PCSK9 gene to treat heterozygous familial hypercholesterolemia (HeFH), atherosclerotic cardiovascular disease (ASCVD), and uncontrolled hypercholesterolemia. Impressively, the base editing platform managed to progress from bench to bedside in only six years.
Future considerations
Gene editing is without a doubt an exciting technology and the impressive phase III and long-term data from Vertex and CRISPR Therapeutics will likely lead to regulatory approvals. To become also commercially successful, some remaining challenges must be overcome, highlighted in Figure 3:
- Safety and ethical concerns: Gene editing technology including CRIPSR/Cas9 can have off-target effects, i.e., that unwanted edits in other areas of the genome can occur. Moreover, this raises ethical issues particularly when the germline is affected, and changes will be inherited by future generations.
- Manufacturing complexity and scalability: Both viral vectors and lipid nanoparticles are employed in the field. Viral vectors are hard to manufacture and scale up when doing larger trials or commercialising. CRISPR innovators will face the choice whether to invest in in-house manufacturing capacities or to work with a contract development and manufacturing organisation (CDMO).
- Price and payer concerns: Gene editing therapies will be priced similarly as other one-off potentially curative treatments. Though such treatments are potentially cost-effective compared to lifetime cost of treatment, the high onetime price tag will remain a barrier. Even more so when the eligible patient population is large.
- Geographic scope: There are two major areas with a high burden from SCA; the US and sub-Saharan Africa. However, due to the high cost, specialist centres required for treatment and manufacturing complexity of Exa-cel, it is likely only patients in the US will be able to receive the treatment, excluding large numbers of patients in sub-Saharan Africa.
- Competitive environment: Exa-cel will have to compete not just against the standard of care but also against bluebird bio’s gene therapy lovo-cel in SCA and Zynteglo in beta thalassemia.
The story around the CRISPR babies of Chinese researcher He Jiankui raised public outcry around the safe and ethical use of the technology. Going forward, these concerns must be addressed by e.g., thoroughly examining ethical considerations by scientists, HCPs, patient groups and religious groups.
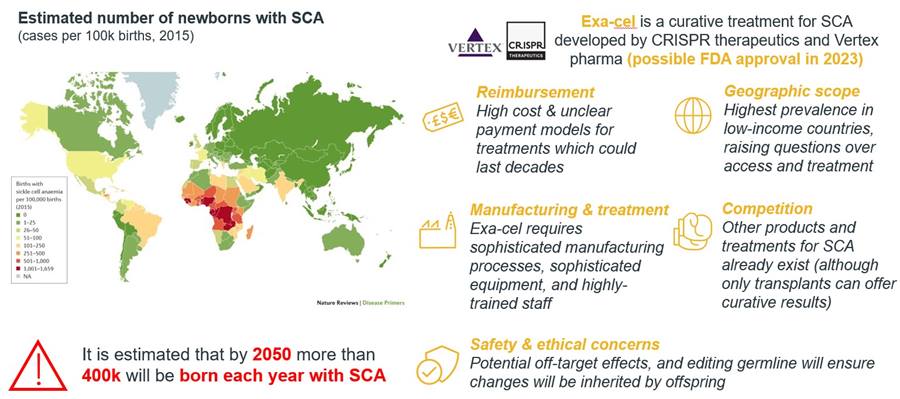
Figure 3: SCA and Exa-cel.
Source: IQVIA EMEA Thought Leadership, Global burden of sickle cell anaemia in children under five. PLoS Med. 10, e1001484 (2013).
Gene editing innovators must take lessons from the early days of cell and gene therapies to ensure that their transformative products reach patients. Companies must engage with payers and healthcare system stakeholders early to ensure patient access and reimbursement. Furthermore, gene editing products must prove their value to both patients and healthcare systems alike. For example, by having a clear evidence strategy that includes RWE collection to show the treatment is effective under real-world conditions. Cost effectiveness will be key. Vertex estimates lifetime treatment costs of SCD can range between $4 and $6 million. ICER already made a case for Exa-cel, saying it would be cost-effective at around $2 million per dose7.
Gene editing with Exa-cel will happen at specialised treatment centres. Patient referrals and long travel times were issues for CAR-T therapy uptake in the past. Vertex, CRIPSR and other pioneers can take lessons to heart by building a network of centres nearer the patient, ensure health system readiness and addressing infrastructure bottlenecks.
Gene-editing therapies hold enormous potential to treat a wide array of diseases and whilst challenges remain, the field is rapidly evolving and IQVIA’s Thought Leadership team will closely watch these developments as they are happening to see whether the pieces are falling into places for gene-editing as a therapeutic platform.

























