Specialized expertise and customized solutions across 14 therapeutic centers of excellence, including oncology, GI/NASH, pediatrics, neurology and rare diseases.
Immunology is the second largest therapy area by value and forecast to reach $156 billion globally by the end of 2023, at ex-manufacturer prices. Over the past 5 years, immunology enjoyed stellar growth at 15% CAGR, or over 3 times the rate of the overall pharmaceutical market. It has given rise to numerous blockbuster brands which harnessed the potential of multi-indicationality (a ‘pipeline in a product’), most notably AbbVie’s Humira, which generated over $20 billion in annual, global revenue at list price.
However, overall immunology growth is expected to slow down dramatically over the next 5 years to 4-6% CAGR (2022-2027), as the underlying business fundamentals are disrupted while innovators will have to navigate a much harsher competitive environment (see Figure 1).
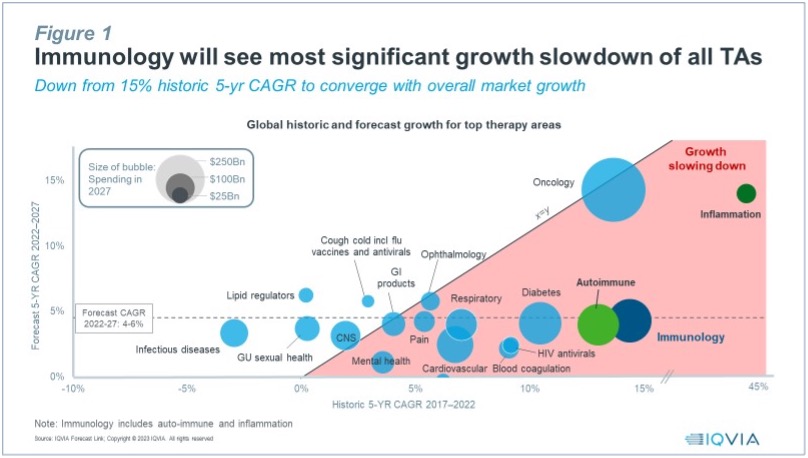
Within immunology, it is important to note the starkly different growth prospects of the underlying autoimmune vs. inflammation market. Future growth of the larger ($132 billion in 2022), more mature autoimmune market is forecast at ~4% CAGR (2022-2027), while the smaller ($18 billion in 2022), less developed inflammation market is expected to grow at 3-4 times that rate, i.e., 12-15% CAGR, over the same time period.
Autoimmune: From hyper-growth to growth pocketsLong-dominating, first-generation innovation in the autoimmune market is maturing, as major flagship brands are facing LoE. Especially the entry of biosimilars of leading brands Humira, in the U.S., and Stelara will introduce low(er) cost options for two backbone mechanisms of action, anti-TNF and IL-12/23, across major autoimmune indications, including RA, IBD, PsO, PsA. Those biosimilars will exert downward price pressure beyond the originators to erode top-line market growth and will have profound implications for sequencing of therapy options, as payers seek to push biosimilars for first-line use, thus relegating newer, innovative therapies to later lines.
At the same time, all major autoimmune indications face extensive crowding as the next wave of innovation is jostling for position, with intensifying inter- and intra-class competition as therapy options proliferate. For example,
-
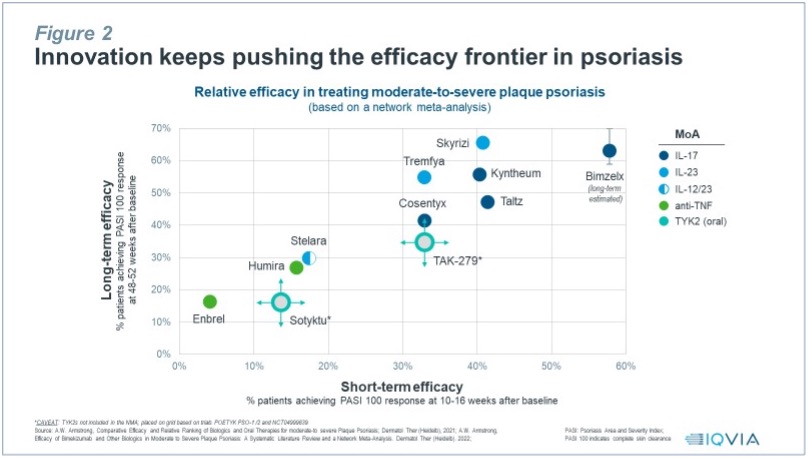
Psoriasis has become the most crowded of all autoimmune indications, with fierce competition between six major drug classes, including anti-TNF, IL-12/23, IL-17, IL-23, PDE4 and TYK2, and among several brands within drug classes, e.g., four IL-17 inhibitors Cosentyx, Taltz, Kyntheum, Bimzelx, three IL-23 inhibitors Tremfya, Ilumya, Skyrizi, or several anti-TNF inhibitors and their biosimilars. While only one TYK2 inhibitor has entered the market to date, BMS’s Sotyktu, half a dozen TYK2 pipeline assets are already in phase 2, e.g., Takeda/Nimbus TAK-279, Pfizer/Roivant brepocitinib, or Ventyx VTX958, with several others in earlier stage development.
Finding differentiation is increasingly challenging in psoriasis as innovation keeps pushing the efficacy frontier, with PASI 100 (i.e., full skin clearance) now the benchmark to beat (see Figure 2).
- Inflammatory bowel disease is at the cusp of facing the level of extensive crowding already seen in psoriasis. Over a dozen late-stage assets are in development for both Crohn’s disease and ulcerative colitis, spanning multiple new mechanisms of action, e.g., JAK, TYK2, S1P, IL-23, TL1A and more, with several products likely to enter the market in each class. This will create complexity and fragment the treatment landscape which is currently dominated by several anti-TNFs and their biosimilars, an integrin (Entyvio) and an IL-12/23 inhibitor (Stelara), with different competitive dynamics playing out in Crohn’s disease vs. ulcerative colitis.
Such abundance of choice gives payers more leverage to extract value, as illustrated, for example, in the widening gross-to-net gap in the U.S. In 2022, average off-invoice concession by manufacturers exceeded 50% in immunology for the first time ever.
Innovators will need to carefully carve out their place in increasingly complex treatment algorithms, with high-stakes head-to-head data becoming critical to demonstrate superiority vs. leading competitors and/or the SoC to convince payers and HCPs, combined with RWE to pin-point patient segments of high unmet need who benefit the most from a given therapy.
Furthermore, the autoimmune market continues to be fiercely competitive, with total promotional investment exceeding $3 billion p.a. globally in each of the past 5 years, while in the U.S. market leading autoimmune brands consistently feature among the top 10 spenders on TV advertising and other direct to consumer (DTC) activities. Consequently, playing in the autoimmune market and rising above that noise level comes at a heavy cost to innovators.
Autoimmune innovators therefore face an unforgiving environment – squeezed between new low-cost options and a crowded innovation frontier. Such opportunity fragmentation also spells the likely end of the possibility of any future $10 billion+ autoimmune mega-blockbuster.
As white space in major autoimmune indications is disappearing, it is not surprising that innovators are increasingly looking elsewhere for new opportunities:
- In smaller autoimmune indications with high unmet need and no/few available treatment options, such as lupus, hidradenitis suppurativa, alopecia or generalized pustular psoriasis.
- In inflammation which, as noted earlier, is a less mature market than autoimmune, with many indications of high unmet need, e.g., atopic dermatitis, asthma, eosinophilic COPD, urticaria, eosinophilic esophagitis or prurigo nodularis. Over the past 5 years, the inflammation market has spectacularly taken off and increased sixfold in value to reach $18 billion globally in 2022. In terms of maturity, this market is 5-10 years behind autoimmune and can still support a mega-blockbuster (Dupixent), possibly two, but competition is intensifying too, especially in atopic dermatitis and asthma, as numerous innovators are attracted to the inflammation opportunity.
Two key trends are shaping the emerging innovation landscape and future competitive dynamics in immunology:
-
Beyond injectables: Immunology is dominated by biologic therapies, many of which have proven to be highly efficacious, and safe, but inevitably require injections, while some are still given as infusions. Innovators therefore seek to reconcile superior, biologic-like efficacy and safety with the convenience of a pill, or to a lesser extent a topical formulation.
Over the past 18 months, 4 oral and 3 topical new therapies were launched across psoriasis (Sotyktu/oral; Vtama, Zoryve, both topical), ulcerative colitis (Zeposia, Rinvoq, both oral) and atopic dermatitis (Opzelura/topical; Rinvoq, Cibinqo, both oral), with more to come: Over a dozen oral or topical late-stage assets are currently in the immunology pipeline, including many new mechanisms of action, e.g., TYK2, S1P, TLR9, MiR-124, plus new JAK inhibitors and novel oral inhibitors of the proven targets IL-17 and IL-23.
Those oral treatments will join biosimilars in the scramble for first-line therapy, with the winner likely to be determined by payer considerations. -
Precision medicine: Two TL1A inhibitor assets currently being investigated in ulcerative colitis and Crohn’s disease, Merck/Prometheus’ PRA023 and Roivant’s RVT-3101, are using (yet-to-be-disclosed) biomarkers for patient stratification in their respective clinical trials. Recent readouts from two phase 2 trials in ulcerative colitis have been encouraging, with biomarker-positive populations achieving better outcomes:
- In the ARTEMIS UC trial, treatment with PRA023 led to placebo-adjusted clinical remission of 37.5% in biomarker-positive patients vs. 25% in all-comers after a 12-week induction period.
- In the TUSCANY-2 trial, at week 56, treatment with RVT-3101 led to clinical remission of 43% in biomarker-positive patients vs. 36% in all-comers, and endoscopic improvement of 64% vs. 50%, respectively.
While more information is needed to understand the wider applicability of such biomarker-based approach, especially its feasibility in routine clinical practice, above examples may signal the arrival of precision medicine in immunology.
These innovation trends are also reflected in recent high-profile immunology deals:
- Merck’s $11 billion acquisition of Prometheus to gain access to the above TL1A inhibitor PRA023 and its potential in precision immunology
- Takeda’s $4 billion acquisition of Nimbus, centred around oral TYK2 asset TAK-279
- Lilly’s $2.4 billion acquisition of DICE Therapeutics to gain access to two oral IL-17 inhibitors, DC-806 and DC-853
- While Roche is said to be in discussions to acquire Roivant’s TL1A inhibitor RVT-3101, also with potential in precision immunology, for a purported $7 billion.
The immunology market of the past is a foreign country, as new business realities take hold. Innovators must therefore move with the times to find success in a dramatically altered, much harsher and unforgiving environment.
Specifically, winning in crowded immunology indications requires:
- Comparative evidence front and centre to demonstrate differential, patient-relevant value vs. competitors – head-to-head and in the real world. This means planning early and comprehensively for a superior, integrated evidence strategy that delivers competitive levels of evidence – both in terms of quality and quantity to rise above the crowd.
- Early medical engagement to carve out a place in complex treatment algorithms, by building advocacy, shaping guidelines and enabling HCPs to navigate an increasingly complex and fragmented treatment landscape. Such early engagement is also critical to gather intelligence on decision making structures, patient journeys and care pathways in each target indication to inform both evidence plans and the overall brand strategy.
- Differentiation beyond the product itself via patient support programmes that deliver a unique patient experience in addition to facilitating optimal clinical outcomes to gain an edge and stand out in a highly crowded market.

























