Gain high value access and increase the profitability of your brands
The calendar page turned from 2023, and we all breathed a sigh of relief. Last year was full of challenge for the pharmaceutical industry as post-pandemic hangovers impacted the industry on top of high inflation, major policy changes with the Inflation Reduction Act, and a continued challenging launch environment. Looking forward in 2024, many of the dynamics from 2023 will persist while several new trends emerge.

-
The Inflation Reduction Act (IRA) – The impact of the IRA continues to dominate as the largest trend in the industry. A true “Before & After” event, the passage of the IRA will reshape life sciences for the next two decades and its influence on U.S. society is just getting started. As the industry moves on from the uncertainty of how to react, we can anticipate that Medicare Part D payers will begin to exert higher levels of formulary control in preparation for 2025 when the new cost distribution models shift upwards of $40 billion annually to payer responsibility. A major offset for this shift in costs is the rebates and fees received from drug manufacturers.
Many manufacturers have examined how the IRA impacts their products and portfolios, and are beginning to adjust to the new economic reality for the next decade. Portfolio rationalization to reduce marginal assets combine with R&D acceleration for key molecules designed to bring indications earlier in the lifecycle. While 2024 may not see these shifts manifest in how drugs enter the market, it will begin to shift pipeline prioritization.
Strategies of the last two decades of indication sequencing are replaced with indication stacking, and more emphasis on evidence throughout a product lifecycle takes increased importance as value conversations come front and center.
IQVIA has published extensively on the impact of the Inflation Reduction Act and will continue to do so as implementation occurs.
-
Obesity Shakes the Market with the success of recent GLP-1 launches. Wegovy, Mounjaro, and Zepbound provide examples of effective launch strategies that will shape how products come to market for the next decade. True examples of post-pandemic modern launch, these introductions combined high patient activation, evidence utilization, celebrity endorsements, social media success, and other novel strategies in their paths to success. There are many lessons to be learned from the GLP-1 class.
However, the obesity launches are not without controversy. Questions of on-label/off-label promotion; the role of the manufacturer in patient support; payer concerns of letting the proverbial ‘genie out of bottle’ with exploding costs for what many consider “lifestyle” drugs; and ongoing questions about maintenance of weight loss and side effect management, are just a few of the issues facing the class.
While weight loss happens early in treatment, health benefits such as reduced cardiovascular events, delayed or eliminated onset of diabetes, and improved respiratory health are often realized years later. Payers must take on the upfront cost for the promise of downstream savings, which can occur much later. Yet the long-term impact on a healthier population and the resultant economic ripples provide much promise.
Regardless, expect to hear much more on the class in 2024. IQVIA is currently tracking well over 100 drugs in various stages of development across manufacturers of all sizes. While we’ve already seen several extremely successful launches, the percentage of patients that are being treated remains under 10% of the potential market. There is a long runway for growth.
-
Pharmacy Biosimilar Adoption will continue to be watched closely by many stakeholders across the life sciences. In 2023, innovators successfully combined the rebate wall created by spread pricing of high list/high rebates with a variety of channel tactics to slow early biosimilar adoption. Despite 12 biosimilars entering the market for adalimumab, some with as much as an 85% discount to list price for the reference Humira, their combined market share remained around 1% at the end of 2023.
Recent news from several of the large pharmacy benefit managers (PBM) indicates that the rebate walls may be coming down, or at least lowered, as net prices come down. The vertical integration of the PBMs and specialty pharmacies accelerate their ability to move share – when they decide to do so. Expect rapid conversion for formulary preferred interchangeable biosimilars that move through vertically integrated systems.
While the U.S. market may not operate on a single payer system, there are increasingly systems within it that are single payers. The control exerted through vertical integration now extends from payer, to Group Purchasing Organizations (GPO), to pharmacy, to prescriber. Those networks will be active in switching high list, non-preferred innovator products to the lowest net cost, or more profitable, products in 2024.
-
Modern Launch challenges continued in 2023 as payer coverage, physician adoption, and patient activation have lagged behind historical trends. After removing vaccines and GLP-1s from the last five years of launch, the average first year revenues for launch products reimbursed under the pharmacy benefit have dropped from $140M in 2018 to $60M in 2022. This is in direct relation to increased payer control and the use of pharmacy exclusions for launch brands.
Manufacturers have increasingly stepped in to subsidize patient demand with transition assistance programs. These programs are designed to provide an easy path for a patient to adopt a brand successfully and to smooth the prescriber experience by removing payer hurdles. The growth of these programs has been significant, with over 50% of year one demand now being fully subsidized by the manufacturer.
In 2024, understanding the intersection of patient activation, the influence of transition assistance on HCP adoption, the true cost and timing of access, and the long-term impact of trying to deconstruct generous manufacturer assistance will be top of mind for any product launching this year.
Evidence supports, and IQVIA continues to believe, that the launch window is no longer the historical norm of 12-18 months but is now closer to 18-36 months, dramatically changing the investment required for success.
-
2024 Electioneering is already starting. One could argue that the 2020 election never stopped, as former President Donald Trump and current President Joe Biden have long held the pole positions as respective party nominees for this year’s election. Expect the rhetoric to heat up even more as we move closer to the election with negativity from both sides pointed at the life sciences industry. Both candidates have battled with pharmaceutical manufacturers and PBMs during their tenures.
It is not a coincidence that the pricing of the first 10 drugs selected for Maximum Fair Price negotiations will be published two months before the election. With large brand names selected, there are many seniors on those products that will be targeted with messages about lower drug costs brought by the IRA under President Biden. The IRA will emerge as his signature legislation during this term in front of the election.
-
Accumulators and Copay Maximizers are bad for patients. Disruptions to care, higher costs for those who can least afford it, and serious concerns about the ethics and marketing of these programs led the U.S. District Court of Washington, D.C. to rule that Accumulator Adjuster programs be disallowed, and that any spend by or on behalf of a patient be allowed to count towards out-of-pocket limits and deductibles for drugs with no generic or bioequivalent. The ruling is not without controversy, and it was only recently that the Biden Administration dropped its appeal, leaving the ruling in place.
Additionally, in late 2023, the Centers for Medicare & Medicaid Services (CMS) published its Patient Protection and Affordable Care Act and the HHS Notice of Benefit and Payment Parameters for 2025, which includes proposed provisions on what qualifies as Essential Health Benefits (EHB). The proposed rule would require all drugs covered on a formulary to be treated as an EHB. This would effectively close the loophole that is being used by PBMs to discount drug spend that is classified as “non-essential health benefit” from counting towards deductibles and out of pocket. CMS is currently seeking comments on the proposed changes.
With an IQVIA estimate of $6-8B in copay spend moving through Accumulator Adjusters and Copay Maximizers, removing them from the market in short order would seriously disrupt in-year benefit designs and economics. As such, do not expect the programs to be removed in 2024, and watch for more litigation to occur.
If these programs do come out of the market, while a win in the effort to lower patient out-of-pocket costs, PBMs will look to recoup those costs elsewhere. Whether through higher premiums, increased rebates and fees, increased utilization management on specialty products, or finding other pockets within vertically integrated models to squeeze, the costs will ultimately be shifted.
-
Demonstration of Value Evolves as launch pressures converge with the Inflation Reduction Act to squeeze the economic lifespan of drugs in the U.S. market. While there are multiple strategies emerging to address components of these market dynamics, a common and effective strategy is to increase the amount of evidence being generated. This extends beyond clinical studies and includes Health Economic Outcomes Research (HEOR), Real World Evidence (RWE), Patient Reported Outcomes (PRO), and truly integrated combinations.
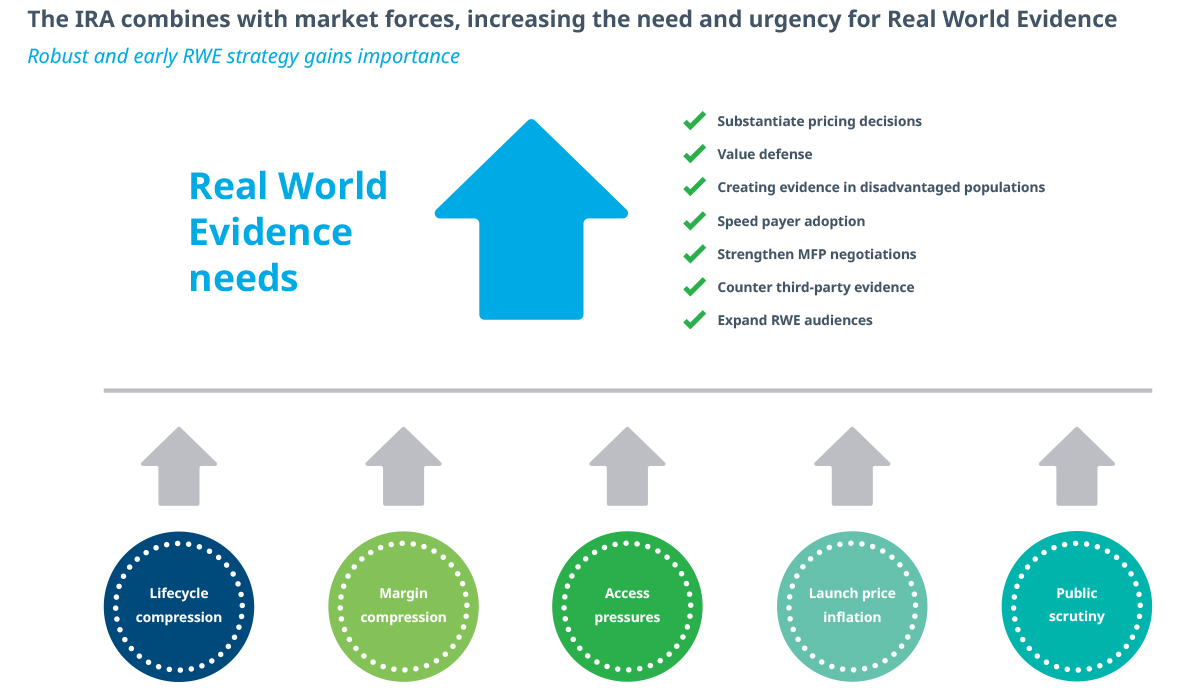
Stronger evidence throughout the product lifecycle can be used to substantiate pricing decisions, counter third-party assessments, create evidence in disadvantaged populations, speed payer adoption, strengthen MFP negotiations, and expand audiences beyond the traditional medical community. A great example of the latter is how evidence on obesity drug efficacy has been reported by news outlets ranging from The Wall Street Journal and The New York Times to People magazine.
In 2024, the entire industry will continue to reevaluate investment into all forms of evidence. Early pre-launch investment matters, as it can take years to create the weight of longitudinal evidence required for success in modern markets. On average, drugs that achieved Launch Excellence have twice the evidence within the first 24 months as all other launches.
-
Cracks in the System Widen in 2024 as post-pandemic access to care, market economics, and skinnier drug formularies pressure patients and drug utilization. The last three years have seen a dramatic increase in the use of cash pay options and drug discount cards like GoodRx, the emergence of telehealth like Hims/Hers, and the creation of discount pharmacies like Mark Cuban’s Cost-Plus Drugs.
Patients are choosing to use these channels instead of going through traditional market models. Many times, that is because it allows more favorable pricing than insurance or because access is otherwise restricted. With 8.5% of the total U.S. drug universe moving through the cash channel, up from a pre-pandemic 5%, that means that there are now over 500 million prescriptions filled outside of insurance.
The Inflation Reduction Act will contribute to narrowing of formularies as payers struggle with shifting costs in the Catastrophic Phase of coverage beginning in 2025. This will push more patients into the cash market, growing the channel overall in 2024.
Beyond formularies, the closing of retail pharmacies by CVS and Walgreens, combined with the Rite-Aid bankruptcy, will impact direct and physical access to care at retail pharmacies. Many of these store closures are happening in communities that already were lacking access and will further exacerbate real structural health equity challenges. Given the importance of access to care at local pharmacies, anticipate this to work its way into the 2024 election.
-
Pricing Pressure and Plateaus have already begun to stabilize price increases across the industry. Since 2017, the annual net price increase for drugs has been at or below the rate of inflation. Many pricing practices of the past have been widely discarded. Gone, too, are the large price increases that often followed mergers and acquisitions of legacy assets.
Inflation penalties now include both government channels in Medicaid and Medicare and are common practice in PBM contracts (price protection) across all payer channels. In the Medicaid channel, the removal of the penny pricing cap now means that brands can face negative pricing in the channel. Following the insulin price reductions in 2023, expect that manufacturers with products with negative net pricing in Medicaid will evaluate, and sometimes pursue, strategies to decrease list price. Negative pricing on drugs will begin to alter purchasing patterns, as stakeholder move from a motivation of cost avoidance to one of profiteering or seeking agents with the largest negative margin.
Ironically, the combination of price controls will place pressure on manufacturers to increase their list price at launch. Net price pressure throughout a brand’s lifecycle is a complex combination of discounts, and list price had been an important strategic lever to combat those. Without the ability to address price over time, there will be pressure to launch at higher prices to account for the inevitable net price decline that all manufactures feel as products move through the maturity phase.
-
Persisting 340B Challenges will continue to impact the supply chain, manufacturer net prices, and insurance premiums. Despite moves by nearly 30 manufacturers to limit the 340B pharmacy network, channel growth has pushed total sales to over $100M. While program utilization has slowed in the retail channel, it has broadly accelerated elsewhere, more than offsetting manufacturer mitigation.
Manufacturers will continue to carry the burden of scrubbing out duplicate discounts, looking for increased transparency into the program, and seeking changes to the system. Unfortunately, 340B regulation and transparency do not sit high on the policy radar, so do not expect relief anytime soon.
New questions on the true impact of the 340B program are emerging as responding to the economics at play is like going down the proverbial rabbit’s hole in Alice in Wonderland. Perverse economics, system incentives, 340B patient definitions, product diversion, for-profit hospital utilization, and more all call into question the ability for any meaningful reform to occur.
As the Inflation Reduction Act gets implemented, exclusions for 340B from CPI penalties and MFP discounts will place a new seat at the table for CMS. The expectation that CMS can use product modifier codes embedded in claims data to manage 340B utilization will fall short, as only a small percentage of claims carry the voluntarily added fields.
Summary
With a few more positive trends on the horizon than we have seen in the past, success is still possible in 2024 and over the next decade. Navigating the Market Access space in the U.S. remains complex and can be summarized into two major dynamics – the compression of the economic lifecycle and a narrowing of the access funnel.
The compression of the economic lifecycle is the combination of many forces like the IRA, Modern Launch, and pricing pressures. Narrowing of the access funnel is happening as formularies narrow, payers take on added costs, physical access to care decreases, and post-pandemic system pressures, such as provider burnout, reduces capacity.
Addressing these two major trends requires a detailed understanding of how the myriad dynamics combine at the patient, provider, payer, and pharmacy levels. Rebuilding clinical development strategies to pull value forward into the lifecycle, focusing investment on rapid growth strategies, and removing marginal ROI spend are more critical than ever before. One major strategy, consistent across all the Top 10 U.S. Market Access Trends for 2024 is the evolving utilization of evidence to determine value and to understand how these dynamics impact patients.

IQVIA’S Market Access Center of Excellence
Related solutions
Data, AI, and expertise empower Commercial Solutions to optimize strategy, accelerate market access, and maximize brand performance.












