Rely on market-leading strategists to help you achieve maximum access and profitability
















- Locations
- United States
- US Blogs
- Inequalities in Access to Treatment for Type 2 Diabetes Patients with Chronic Kidney Disease
In March 2022, IQVIA published The Impact of Payer Access in Chronic Kidney Disease (CKD), and now continues its investigation of CKD patient access with a look into the impact of payer controls on those most affected in this disease area. Payer controls in the form of restrictions and cost-sharing can impact prescribing behavior, but are also contributing factors as patients make final decisions at the pharmacy. To understand the full influence of payer controls, it is valuable to examine the final hurdle that emerges after the patient has sought out a provider, been prescribed treatment, and arrived at the pharmacy.
According to the Centers for Disease Control, CKD is more common among people over 65 and among Black and Hispanic adults1. Moreover, the mortality rate from kidney disease among Black individuals is twice that among White individuals2. This discussion focuses on findings from a recent IQVIA study to determine whether treatment barriers inadvertently and disproportionately impact different demographic groups.
Percentage of adults aged 18 years or older with CKD
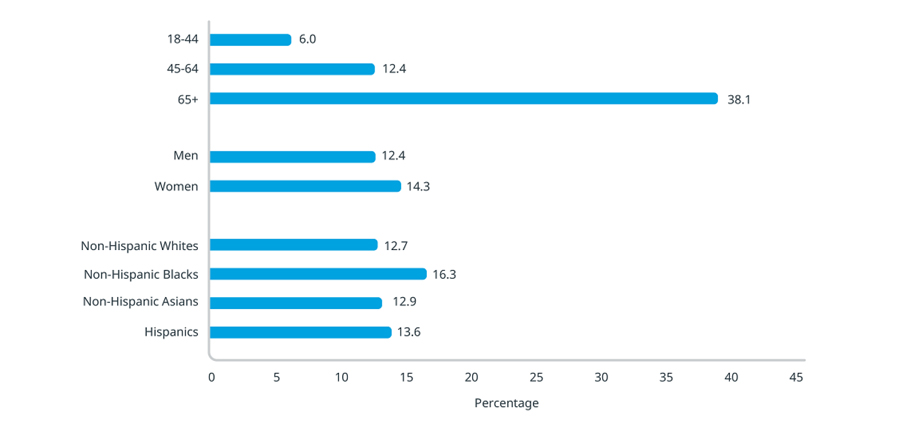
Source: CDC – Chronic Kidney Disease in the United States, 2021
https://www.cdc.gov/kidneydisease/pdf/Chronic-Kidney-Disease-in-the-US-2021-h.pdf
Insurers can follow different approaches to reviewing newly approved treatments before putting them on formulary, which can sometimes delay coverage. An analysis of pharmacy claims for Kerendia (finerenone), a treatment from Bayer for CKD in Type 2 Diabetes, found that the 30-day new patient rejection rate for Medicare Part D patients was 66%, 37 percentage points higher than commercially insured patients (29%), across the first nine months post launch. It is important to note that across payer channels, especially in Medicare Part D, most of the rejections are attributable to lack of coverage, which tends to be the most enduring rejection type when compared to prior authorization or step therapy requirements.
Kerendia new patient utilization management by payer channel
(30-day look-forward, July 2021 - March 2022)
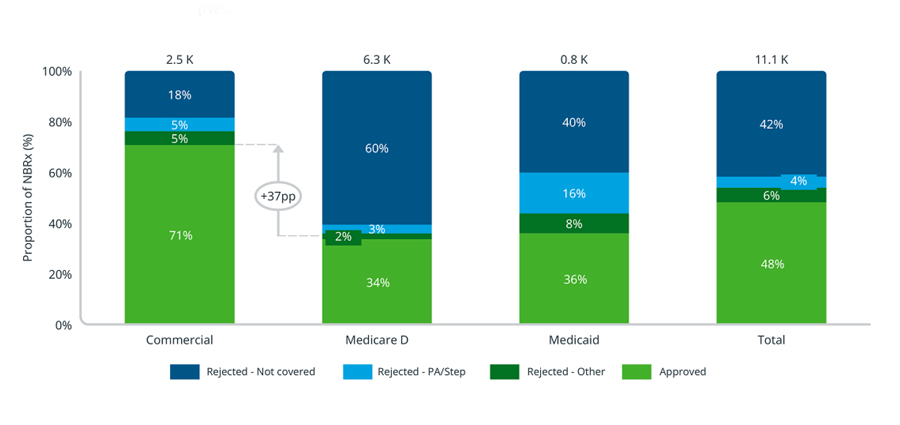
Source: IQVIA Longitudinal Access & Adjudication Data (LAAD) pharmacy and medical claims data of Type II diabetes patients diagnosed with CKD, July 2021 – March 2022
Notes: Utilization management looks forward 30 days after a patient’s initial attempt to account for any rejections that the patient may have been able to overcome.
A key factor in reducing the societal impact of CKD in Type II diabetes (T2D) is equal access to treatment for all patients. Across payer channels, there were no notable discrepancies between patient ethnic groups regarding initial approval rates of Kerendia. However, Black patients were less likely to overcome Medicare Part D rejections within 30 days when compared to other ethnicities. This subgroup of patients only experienced a 21 percentage point increase in their approval rate after 30 days, compared to 25-29 percentage point increases for patients in other ethnic groups.3
Kerendia new patient utilization management by ethnic group in Medicare Part D (Initial vs. 30-day look-forward, July 2021 - March 2022)
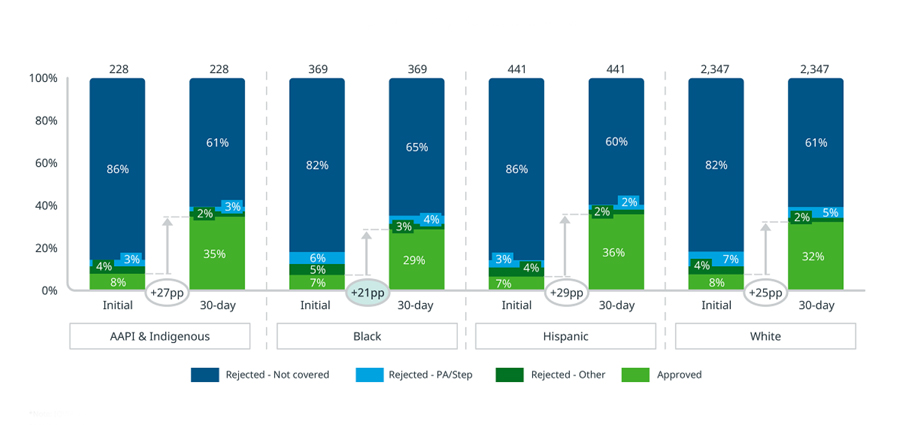
Source: IQVIA Longitudinal Access & Adjudication Data (LAAD) pharmacy and medical claims data of Type II diabetes patients diagnosed with CKD, July 2021 – March 2022
Notes: Utilization management looks forward 30 days after a patient’s initial attempt to account for any rejections that the patient may have been able to overcome. AAPI and Indigenous patient ethnic groups are combined for sample size.
Furthermore, Black patients whose Kerendia prescriptions were rejected are also less likely to end up on an alternative treatment, compared to patients of other ethnic groups. In the 90 days following their Kerendia rejection, 95% of Black patients did not begin treatment with a sodium-glucose cotransporter-2 inhibitor (SGLT2i) compared to 88-90% for other patients. Thus, analyses imply that fewer Black patients may end up on Kerendia or any alternative treatment for their CKD compared to other ethnic groups.
Observed SGLT2i alternative fill following a Kerendia rejection by patient ethnic group in Medicare Part D (90-day look-forward, July 2021 -March 2022)
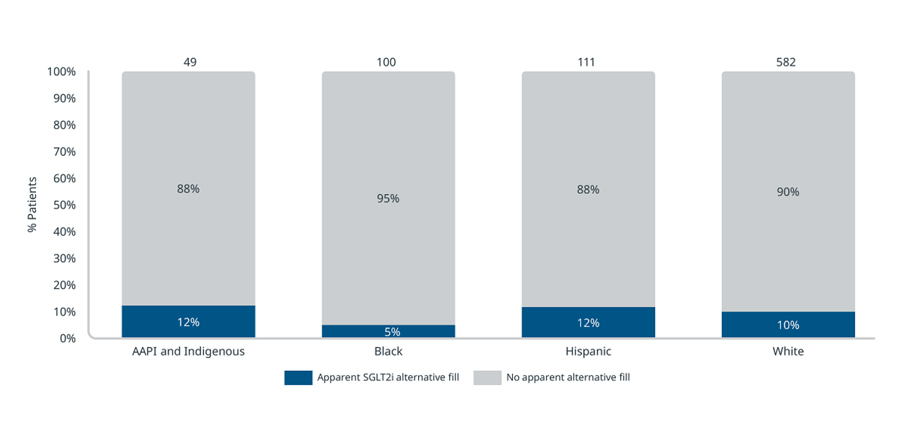
Source: IQVIA Longitudinal Access & Adjudication Data (LAAD) pharmacy and medical claims data of Type II diabetes patients diagnosed with CKD, July 2021 – March 2022
Note: Alternative fills look forward 90 days after a patient’s initial rejection to account for any other indicated treatments the patient may have filled. AAPI and Indigenous patient ethnic groups are combined for sample size.
When patients are able to navigate initial access barriers and are finally approved for treatment, they are then exposed to costs determined by the prescription drug benefit under their insurance plan. As demonstrated in Figure 5 below, prescription abandonment rates are lowest at $0 out-of-pocket costs (i.e., the co-pays or co-insurance assigned to a given medicine within a patient’s prescription drug benefit insurance plan) and increase with patient cost-sharing. Cost sensitivity is observably greatest among Black patients, whose abandonment rates exceed those of White and Hispanic patients at the same out-of-pocket level. In Medicare Part D, Black patients have the highest abandonment rate across all cost cohorts. It is necessary to acknowledge that many factors can influence the observed differences in access and behavior beyond patient ethnicity. Income, education, geography, language, and more can all play a role in the treatment journey of a patient.
Kerendia new patient abandonment by out-of-pocket cost and ethnic group in Medicare Part D (14-day look-forward, July 2021 – March 2022)
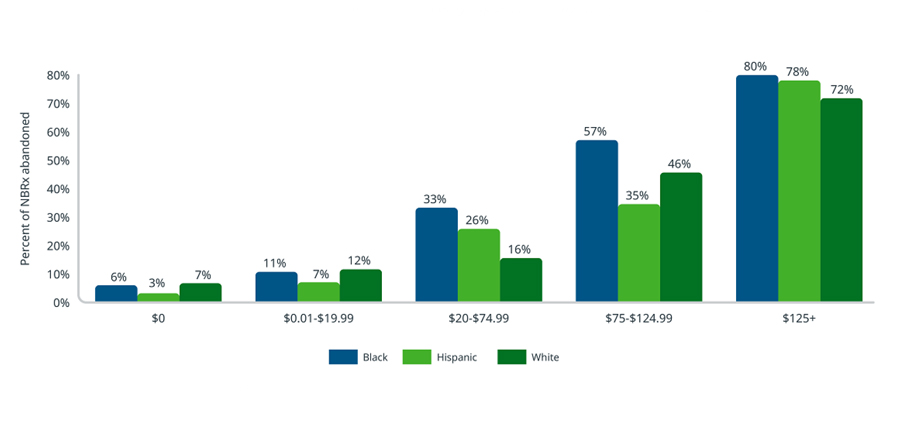
Source: IQVIA Longitudinal Access & Adjudication Data (LAAD) pharmacy and medical claims data of Type II diabetes patients diagnosed with CKD, July 2021 – March 2022
Note: Abandonment looks forward 14 days after a patient’s initial approval to account for any delays before filling a prescription. AAPI and Indigenous patient ethnic groups are excluded from this analysis due to sample size.
Implication
The public health and societal impact of CKD, and associated financial burden, make improving access to care and addressing disparities a particularly urgent concern. On top of declining kidney function, and potential kidney failure and dialysis, with negative impact on quality of life, CKD patients with T2D are reported to have twice as many myocardial infarction events and have an 83% greater risk of cardiovascular related death compared patients with T2D alone. Treating Medicare beneficiaries with CKD in 2019 cost $87.2 billion, and treating people with end-stage renal disease cost an additional $37.3 billion.
Like many disease areas, patient access in CKD is affected by formulary coverage and out-of-pocket costs. In particular, Medicare Part D patients can face greater barriers through delays and higher restriction use. As the industry seeks to improve access – and address disparities in care and outcomes – continued measurement of how these barriers disproportionately impact distinct patient groups is needed.
About IQVIA’s Market Access Center of Excellence
With a passion for innovation, quality, and customer service, IQVIA’s Market Access COE is at the forefront of industry trends in biopharma and therapy access. The COE’s portfolio of services includes patient engagement and commercial solutions that continue to advance a long-standing mission to the enhancement of patient access and health outcomes while delivering an unyielding focus on driving client satisfaction. Market Access is committed to driving real change for its clients and patients by way of newly combined offerings, expertise, and talent from the diverse organizations of more than 1,000 dedicated individuals within it.
Acknowledgements
The authors would like to thank several IQVIA colleagues for their support; in particular, Malin Ortenblad, Eric Savage, and Ingrid Hirt. The authors would also like to acknowledge Bayer for sponsoring the analyses used in this blog. The findings and final editorial decisions belong to IQVIA.
1 Centers for Disease Control, Chronic Kidney Disease in the United States, 2021 https://www.cdc.gov/kidneydisease/pdf/Chronic-Kidney-Disease-in-the-US-2021-h.pdf
2 Benjamins MR, Lorenz P, Saiyed S et al. (2022) Kidney disease mortality across the 30 most populous cities: assessing overall trends and racial inequities. JGIM, (SPI 7444)
3 Wu B, Bell K, Stanford A, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns–NHANES 2007-2012. BMJ Open Diabetes Res Care. 2016;4(1):e000154
4 Papademetriou V, Nylen ES, Doumas M, et al. Chronic kidney disease, basal insulin glargine and health outcomes in people with dysglycemia: the ORIGIN study. Am J Med. 2017;12(130):1465.e27-1465.e39
5 Centers for Disease Control and Prevention. Accessed January 31, 2022. https://www.cdc.gov/kidneydisease/basics.html

Patient Affordability and Engagement Services
Increasing patient access to your brand requires engaging and educating eligible patients and health care professionals on the details of your therapy as well as removing the burden of cost from the patient.
Related solutions
Increase patient access and adherence.
Gain high value access and increase the profitability of your brands
Data, AI, and expertise empower Commercial Solutions to optimize strategy, accelerate market access, and maximize brand performance.




