IQVIA Complete Consent is the user-friendly, flexible, and feature-rich electronic informed consent solution built to support diverse protocols at a global scale






















- Blogs
- Stop Papering Over Consent Problems
An electronic informed consent (eConsent) solution provides multiple benefits to patients, caregivers and clinical trial staff. Yet several barriers still prevent widespread adoption. Concerns about the upfront investment of time, IT buildout and deployment, staff behavior change and skill-building – as well as the cost of the platform itself – remain among many clinical operations professionals.
These are valid concerns, but IQVIA’s decades of experience shows the process efficiencies, improved compliance, reduced reliance on paper forms and strengthened patient engagement and retention deliver a clear return on sponsors’ investment. The most important benefit is the operational efficiencies gained by using eConsent, including:
- Increasing randomization rate
- Reducing protocol deviations, and
- Decreasing monitoring time
Let’s dive into the data behind these experiences to demonstrate their positive impact on trial outcomes.
Examining the Data: Increase in Randomization Rates
For almost all clinical trials, patient recruitment and retention are both key metrics and challenges for executing a successful trial. The number of identified patients to be pre-screened, consented and eventually randomized drop significantly at each stage. However, in reviewing industry academic research based on historic data, by employing eConsent, researchers need 25% fewer patients to reach the same completion goals for a trial.
To validate this, we did our own analysis across IQVIA studies. We found, while comparing the randomization rates for 55 trials that employed eConsent compared to other trials that used the paper consenting process, there was a 22% increase in randomization rates.
What could be the reason for this impact? eConsent increases patient retention, engagement and adherence by:
- Enabling patients to have a better understanding of the risks and benefits of trial participation.
- Offering powerful capabilities to optimize study education such as multimedia, contextual assistance (glossaries, images, etc.) and accessibility features for the visually impaired (zooming in/ audio narration). Some solutions also include special provisions forpediatric patients with age-appropriate language and visualizations.
- Allowing patients to facilitate dialog with the medical staff.
- Providing capabilities to easily consent from home.
How does this translate to financial impact for sponsors? Approximately 30% of patients drop out of clinical trials, resulting in heavy financial costs. On average, it costs $6,533 to recruit one patient to a clinical study, and the average cost of replacing patients in a study is approximately $19,533 per individual. Increasing randomization rates by 22% clearly translates to financial savings.
Reducing Protocol Deviations
eConsent solutions streamline the consenting process for site staff by providing clear visibility into the consent flow. These systems add safeguards to ensure that all steps are followed, amendments are tracked and a clear audit trail is maintained throughout the process. These automated workflows with built-in checks and balances, ensure that site staff meet compliance requirements leading to reduction in consent related deviations.
To prove this, we analyzed 100 clinical trials that employed IQVIA Complete Consent and found that these trials had only 6% deviations as compared to consent related protocol deviations across all IQVIA trials which averaged at 14%. The result was a whopping 57% reduction in informed consent form (ICF) related protocol deviations.
Study-level analysis of consent-related major and critical deviation rates in eConsent vs. non-eConsent usage also showed a significant decrease in eConsent studies. There were 22% fewer major and critical deviations per randomized patient and 53% fewer major and critical deviations per patient visit in eConsent vs non-eConsent studies. We also analyzed nearly 12,000 consent-related deviations across the studies and realized that as many as 90% of costly consent-related deviations can be mitigated or avoided with eConsent.
Almost all major and critical deviations lead to corrective and preventive actions (CAPAs) where resolving these involve significant amounts of time and resources. We conducted site staff and monitor surveys to assess the time spent in resolving CAPAs and determined that overall savings can be derived from using eConsent. For every major and critical deviation that is avoided, a sponsor could save over 224 hours and an average of 67 minutes per patient, as indicated in the below graphic. This was taking into consideration typical scenarios of ICF re-sign including but not limited to subject recall, new subject visit, ICF invalidation, new signature, site documentation and tracking.
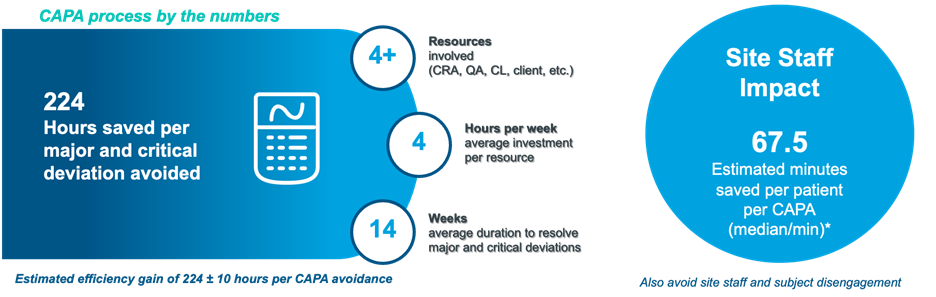
By reducing deviations, there is a direct impact to completeness, accuracy and reliability of the study which eventually impacts trial completion goals and timelines. Patient recruitment speed, dropouts and deviations directly and indirectly contribute to study delays , which lead to wasted time and resources.
Studies have shown that 80% of trials are delayed by at least a month, causing potential losses of approximately $600,000 (and potentially as high as $8 million) per day. By employing eConsent solutions, the indirect impact to patient dropouts and protocol deviations can lead to savings that extend well beyond the overall expenditure on these solutions.
Decreasing Monitoring Time
An eConsent solution ensures that every patient has consented to correct versions of all ICF documents. It tracks that signatures are captured prior to interventional procedures according to a scheduled date and time and an adequate audit trail is maintained throughout the process. In addition, monitors have access to detailed analytics and reports on this consenting data, allowing them to prepare in advance for a more focused monitoring visit, resulting in saving time spent with site staff on site. We found that for every ICF, an average of 15 minutes is saved in monitoring activities, when an eConsent solution is used at the clinical site.
Looking at other eConsent efficiency benefits
Using an eConsent platform improves trial data management including strengthening patient retention and reducing overall costs. Additionally, sponsors benefit from the quality and efficiency of the clinical trial process in five key areas..
- Streamlining trial operations with integrated technologyA clinical trial often employs a vast array of technology solutions to manage complex trial related operations. An eConsent solution integrates disparate digital products and solutions for seamless data transfer across systems for streamlined processing. As a result, site staff save valuable time by no longer replicating data and actions across systems and can provide a more seamless and simplified patient experience.
- Reducing carbon footprint
Digitally, automated consents with workflows ease site burden and reduce the carbon footprint by eliminating printing and shipment through paperless consents and remote monitoring capability.
- Improving data transparency for all stakeholders
eConsent systems use dashboards and reports, to “bubble up” data to visually present progress across consent processes. Relevant data can be aggregated and presented to various stakeholders depending on their function and role. Site staff can be provided specific reports related to patient consents at their specific sites; trial monitors can view information for all sites that they oversee; and sponsors can see globally aggregated data across all sites.
- Assuring global regulatory compliance
An experienced eConsent partner provides a fully validated electronic consent platform backed with knowledge on accepted signature modalities and regulatory compliance requirements that are mandated by global Independent Ethics Committees (IECs), Institutional Review Boards (IRBs) and Health Authorities (HA).
- Lessening consent-related audit findingseConsent increases patient retention, engagement and adherence to trial protocol by enabling patients to have a better understanding of the risks and benefits of trial participation. Creating an engaging, interactive experience also enhances patient compliance which eventually results in fewer consent-related audit findings.
Bringing It Together
Investing in an eConsent solution is a win-win for sponsors, trial staff and patients, so this is a good time for all stakeholders to set aside their old habits and embrace the new modality that can deliver measurable positive results. Whether it is through reducing deviations and dropout rates or improving randomization for greater recruitment, all stakeholders stand to gain by simply adopting modern digital consent processes for their research.





