





















- Blogs
- The UK could become a leader in Digital Health
Digital Therapeutics are patient health applications and connected devices that have proven clinical benefits, as well as, appropriate regulatory clearances and distribution pathways to serve their intended use. Recent reporting by the IQVIA Institute has identified a large, growing number of such apps with the potential to save billions in unnecessary healthcare costs.1 This value drives strong industry growth projections, with some forecasting that Digital Therapeutics sales will expand to $USD 9.4 billion worldwide by 2025.2
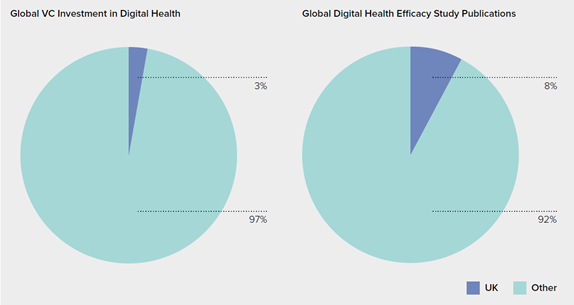
In many ways, the UK is in a position to grow and catch up with the market leaders in the Digital Therapeutics industry. To date, only 8% of published app effectiveness studies have taken place in the UK.3 In 2017, $USD 14.2 billion was invested in digital health startups globally, but only $USD 426 million (3%) was invested in UK digital health startups.4
The UK’s Global Position in Digital Health
If Digital Therapeutics companies could conduct their studies better, faster, and cheaper in the UK than elsewhere, the benefits to the health and wealth of the nation would be significant. Improving the UK’s Digital Therapeutics research infrastructure would position the nation for more investment in research and downstream commercial activity; UK startups would become more competitive, and more world leaders in Digital Therapeutics would want to set up operations in the UK. However, to realise these clinical and economic benefits, the leading apps that drive this value would likely need to be studied in NHS patients prior to their broad adoption.
The research needs of the Digital Therapeutics industry are – at the highest level – fairly uniform and well understood. To be sustainably successful, Digital Therapeutics developers of all kinds need an efficient means of proving their effectiveness, safety and cost-effectiveness relative to the standard of care in a setting that very closely resembles real world practice. In fact, most industry participants would agree that the main problem with current infrastructure is not necessarily ‘quality;’ it’s the excessive time and cost of today’s studies together with the struggle to transition seamlessly from research to real world practice.
A turnkey solution for Digital Therapeutics research
The UK has an opportunity to provide the world’s first turnkey solution for Digital Therapeutics research. Key features of such a solution should include the following:
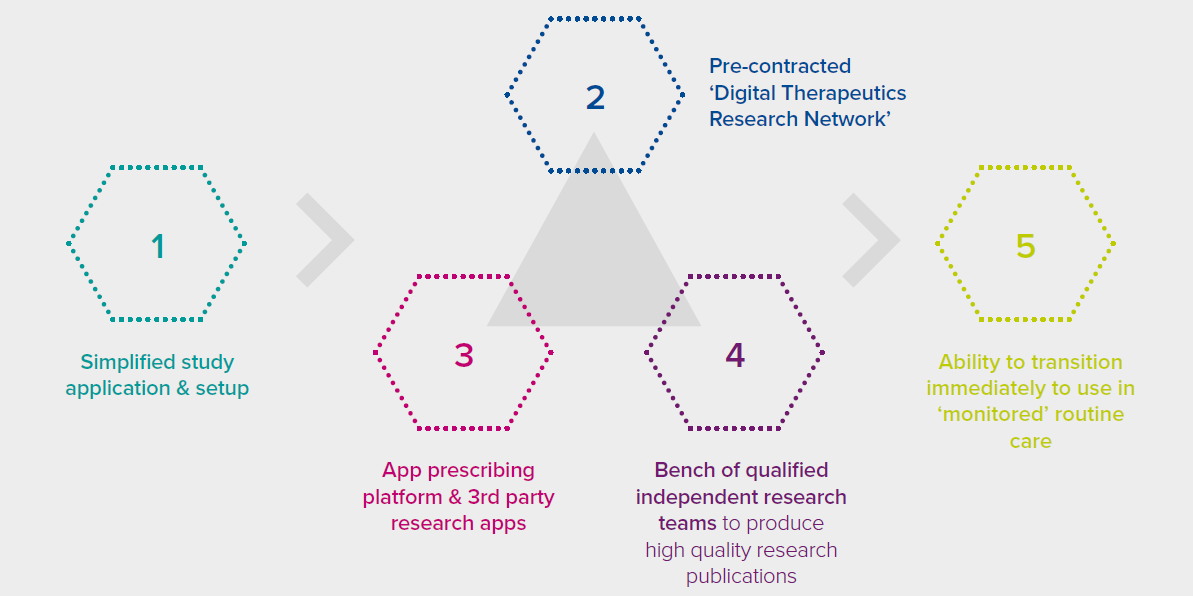
Simplified application & setup
The commonalities of many Digital Therapeutics create the opportunity for a ‘templated’ approach to developing study protocols, drafting ethics review applications, and matching promising studies to potential funding sources.
Pre-contracted ‘Digital Therapeutics Research Network’
It generally takes years for Digital Therapeutics companies to find and set up appropriate legal agreements with suitable research sites. The ability to ‘plug in’ immediately to a network of appropriate sites would be priceless for the industry.
App prescribing platform & 3rd party research apps
Studies should minimise the work required of clinicians and patients. Significant efficiency can be realised by providing healthcare professionals with the ability prescribe study apps from their EMR, by providing patients the ability to eConsent to study terms, and by providing qualified researchers with a single source of collated EMR, app, and other data to analyse.
‘Bench’ of qualified independent research teams
A list of academic and private sector teams with expertise in the management and analysis of pseudonymised data should be available to prepare manuscripts that meet high quality publication standards, such as the WHO’s ‘mERA’ checklist.5
Ability to transition intermediately to use in ‘monitored’ routine care
While research can be improved, perhaps an even larger issue for Digital Therapeutics companies is the need to achieve scale. It would make sense to use a common infrastructure for research and routine care to enable seamless transitions.
A turnkey Digital Therapeutics Research Platform
The required ‘investment’ to make the above a reality is not in new technology as the technology exists already. The required investment to become a world leader in Digital Therapeutics is in collaboration across the public, academic, and industrial sectors. A consensus is needed around new processes for giving the UK’s Digital Therapeutics industry a common sense boost.
References
1 IQVIA Institute. The Growing Value of Digital Health in the United Kingdom. 7 November 2017.
2 Grand View Research. Digital Therapeutics Market by Application. July 2017.
3 IQVIA AppScript Clinical Evidence Database. Data on File. 13 March, 2018.
4 Marc Sluijs. DigitalHealth.Network Deals Database. 15 March 2018.
5 S. Agarwal. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ 2016;352:i1174. 17 March 2016





