Bring trials directly to patients to improve access and engagement, increase quality and shorten timelines.






















- Blogs
- Virtual Trials – A more direct path to patients
Traditional clinical trials pose accessibility obstacles for patients, who often have to travel long distances – as far as 50 miles or more – and make major time commitments for multiple trial-related site visits. This burden to patients can threaten clinical trial enrollment, with less than 5% of patients currently participating in clinical research, 49% of participants dropping out before their study ends, and new therapies taking an average of 10 years to reach the market.
In addition, the U.S. Food and Drug Administration is increasingly requiring more patient diversity in pre- and post-marketing studies, reflecting the fact that ‘medical products are safer and more effective for everyone when clinical research includes diverse populations.
The administrative and financial burden on sites can also prove challenging. Almost half of investigators do one study and never do another.
Virtual trials can address these challenges. They are designed to allow patients to participate from home, with the support of home health nurses. A patient centric approach uses telemedicine to enable patient recruitment, gain informed consent, measure clinical endpoints, and monitor any adverse events from the patient’s home. This provides an innovative and more direct path to patients, taking trials directly to them, and offering a more attractive way for diverse and geographically distant individuals to participate.
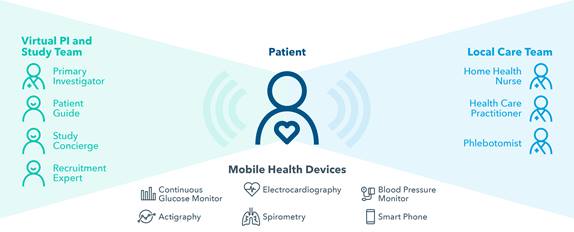
The principal investigator is located remotely and supported by a virtual care team (see diagram). This model provides for better physician oversight and round-the-clock data collection – rather than the infrequent site visits involved in traditional clinical trials. This offers advantages for investigators, who are able to “see” more patients. Moreover, it reduces variability in assessments and data, and provides greater visibility into safety events. Investigators also benefit from technology to support tasks such as issuing alerts and notifications, scheduling, and reporting, freeing up their time to focus on research.
Virtual trials are not ‘one-size-fits-all,’ however. First, it is important to design a patient-centric protocol. The virtual approach works well with chronic diseases and less complex interventional and observational studies. Suitable study types include indications or protocols where the investigational product has a known safety profile and endpoints can be assessed remotely. Initially, therapeutic areas such as endocrinology, CNS, dermatology, respiratory, gastro-intestinal, immunology, cardiovascular, and rare diseases present the best opportunities for a virtual approach.
We should not totally rule out more complex indications where the burden on patients is very high. For these situations and more complex trials, elements of virtual and traditional trials can be combined in a hybrid approach. For example, this might be used to support long follow-up periods after innovative treatments such as cell therapies.
In other areas, virtual trials might not be a good fit. Examples include investigational products with an unknown safety profile, and protocols involving endpoint assessments that have not been validated for remote assessment. Studies where interventions must be conducted in a structured setting such as an intensive care unit or phase I unit – along with early-phase oncology and first-in-human studies – would typically not be suitable for a virtual approach.
In conclusion, by focusing firmly on the patient, virtual approaches can potentially transform the research landscape, enabling better, faster and more efficient trials. They hold particular promise for less complex interventional and observational studies involving chronic diseases. For patients, improved trial accessibility can expand clinical care options, and boost retention. For investigators, the ability to interact virtually with larger numbers of participants provides deeper insights from richer data – and may reduce investigator turnover. Initial indications are that patient and investigator satisfaction is high. However, sponsors should keep in mind that virtual trials involve more than technology alone – they remain a complex endeavor that must meet regulatory requirements and demands in-depth clinical understanding.
IQVIA Virtual Trials is uniquely qualified to orchestrate the unique complexity of patient-centered virtual trials to accelerate the path to approval. Click here to learn more.
----------------------------------------
1E. Miseta. Clinical Leader. July 13, 2015
2Impact Report (2006) Tufts CSDD 8(5)
3J. DiMasi. Tufts CSDD. October 2014
4https://www.fda.gov/ForPatients/ClinicalTrials/ucm407817.htm
5https://www.sciencedirect.com/science/article/pii/S245186541630093X
6http://www.appliedclinicaltrialsonline.com/virtual-clinical-trials-future-patient-engagement

Put Patients at the Center and Accelerate Your Timelines
Benefits of virtual studies
Patient convenience: Patient-centered trials improve engagement and retention, helping to achieve better outcomes
Expanded patient reach and diversity: Access to more diverse patients without geographic constraints — including minorities and difficult-to-recruit populations
Accelerated timelines: Faster start-up, expedited recruitment and better patient engagement lead to shorter overall timelines
Improved quality and safety: Reduced variation in data collection and near real-time data for enhanced safety signal detection
Richer data: Enabling secondary endpoints to be tracked, and providing more evidence supporting post-marketing safety, efficacy and value.
Cost-effectiveness: Reduced costs compared with those of bricks-and-mortar site and monitoring visits; streamlined patient engagement, enrollment, and trial management.
Related solutions
Explore our end-to-end, full service clinical development capabilities including Therapeutics expertise, Development Planning, Phase I early clinical development, Phase IIb/III and Phase IV Trials, Regulatory Submission and Post-Launch Studies.





