Labeling Intelligence Hub
Compare and extract critical drug label information
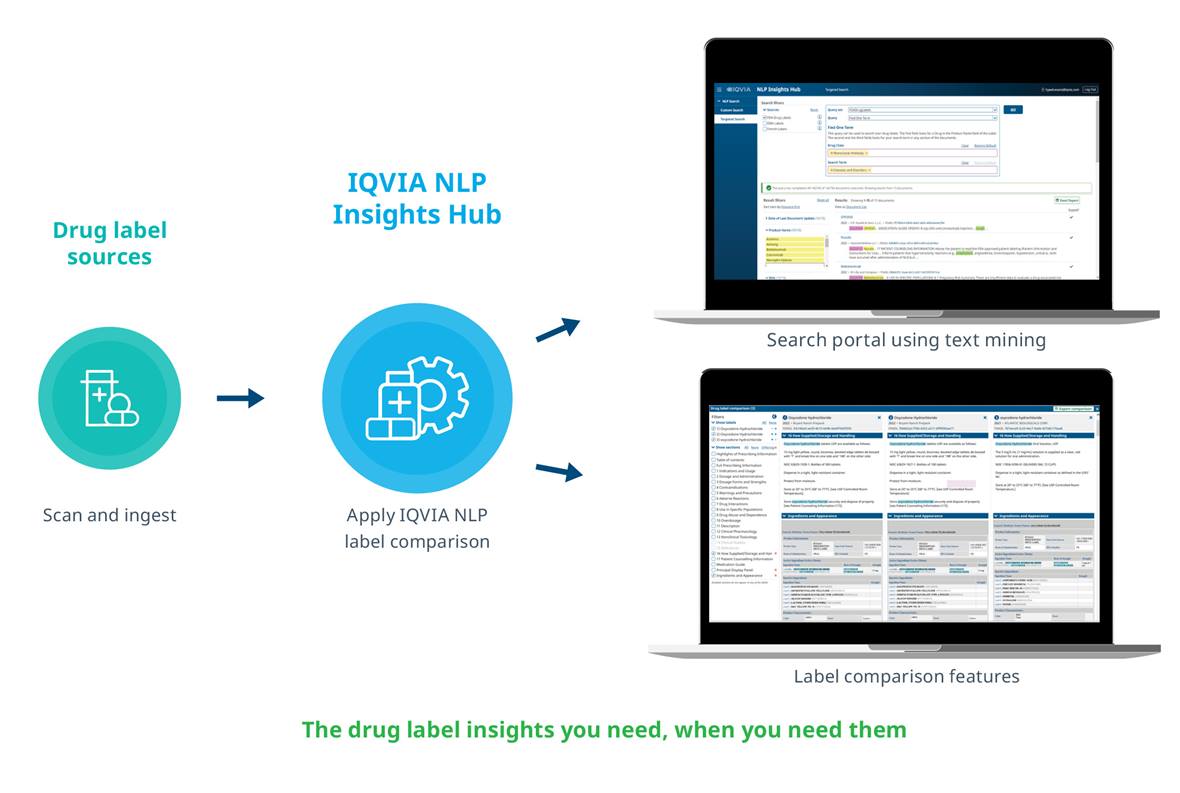
Accurate regulatory intelligence is essential for better decision-making for regulatory teams. The IQVIA labeling Intelligence Hub uses award-winning natural language processing (NLP) to find, extract and compare drug label information with simple and rapid capture of relevant data in labeling documents from diverse sources such as FDA drug labels, EMA drug labels, French drug labels and more.