





















- Blogs
- Advancing Standards of Care in Asthma
The standard of care for asthma is seeing an evolution away from inhaled corticosteroids (ICS) and long-acting beta-agonist (LABA) combinations as maintenance therapy alone, to also include these as “as-needed” therapy, and away from albuterol as-needed therapy alone. This is occurring particularly in the US, UK and European Union.
Asthma affects around 262 million people worldwide, accounting for 461,000 deaths globally in 2019.1 Low- and middle-income countries have the most asthma-related morbidity and mortality.2 There has been significant innovation in asthma treatments over the past 40 years, including inhaled corticosteroids, leukotriene receptor antagonists, muscarinic antagonists and biologics targeting components of inflammation, such as immunoglobulin E (IgE) and mediators of type 2 (T2) inflammation.3 The success of biologics is leading some specialists to consider for the first time the possibility of asthma remission. Several biologics are approved for asthma in the US – omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab – with others in development.4 Nonetheless an estimated 37-88% of asthma patients have uncontrolled disease.5
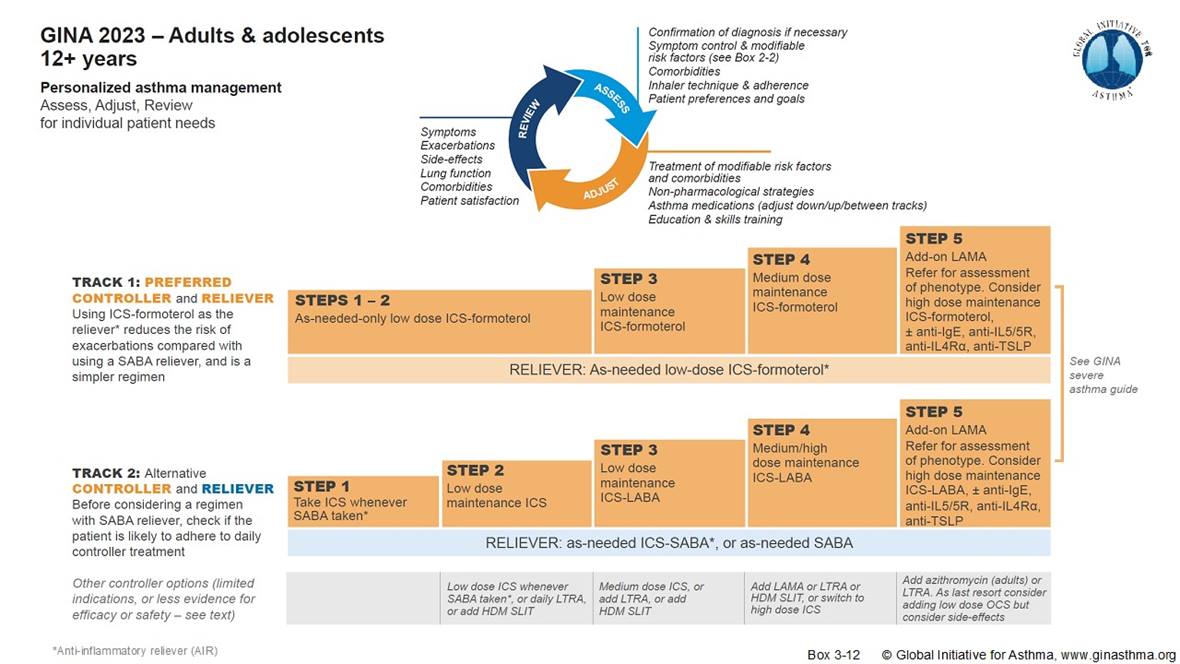
The advancing standards of care in asthma follow a change in the global strategy for management and prevention of this disease from the Global Initiative for Asthma (GINA).6 The widely-used GINA strategy report is a global evidence-based approach that can be adapted for local health systems and medicine availability. In GINA track 1, as-needed ICS-formoterol reliever remains the preferred treatment approach for adults and adolescents (Figure 1). The guidance also describes the management of severe asthma and exacerbations, with recommendations on when to use biologics, ICS-formoterol, and ICS-short-acting beta2-agonist (SABA); there is clarification that GINA recommends biologic therapy for asthma only in severe cases and when any existing treatment has been optimized. Single Maintenance and Reliever Therapy (SMART), also known as MART therapy, is used for patients with moderate-to-severe asthma, comprising a combination treatment of an inhaled corticosteroid and a rapid onset LABA such as formoterol.7 An alternative recommendation is the use of ICS whenever albuterol is used as reliever, either in combination or as separate drugs.
Under the new guidelines, steps 1 and 2 together are covered by health insurance in the UK and some European countries, but not in the US, where regulatory barriers from FDA also exist. There is a pressing need for US coverage too, to enable patients to benefit from new and existing treatment options.
Newer products in R&D may have potential for use in steps two and above in the GINA guidelines, including alternative controllers and relievers.
CRISPR technology has potential to delete allergen genes and may therefore help allergy-related conditions such as asthma.8 This approach is being used in combination with a genomic understanding of the human microbiome to help address asthma. A joint project at UC San Francisco and UC Berkeley is using a $70 million grant from a TED initiative to edit the genes of microbes in the gut and airways that play a role in this and other allergic disorders.9 Gene therapy for the disorder itself is primarily for those involving a single gene, so has less potential for asthma, which is a genetically complex disease.
Clinical development of IL-13 therapies for asthma – including lebrikizumab and tralokinumab – has been halted indefinitely due to insufficient efficacy in clinical trials.10 Dupilumab, which affects both IL-4 and IL-13, has been very effective in severe asthma. A study on specific IL-13 therapies concluded that since IL-13 directly affects airway contractility and mucus production, an anti-IL-13 specific drug with demonstrated efficacy could potentially be included before GINA step 5.
Figure 1: GINA asthma management guidelines for adults and adolescents, 202311
| US Food and Drug Administration: “We note that recent updates to asthma guidelines recommend the use of an ICS/long-acting beta2-adrenergic agonist (LABA) (formoterol) combination product not only as controller treatment, but also as a preferred reliever treatment for some patients. This is a concept known as SMART – single maintenance and reliever therapy; however, no ICS/LABA product is currently FDA-approved for PRN [as needed] use. FDA does not make recommendations regarding asthma guidelines...”12 |
In conclusion, the standard of care for asthma is changing for the better, with new guidelines encouraging approaches that are safer, easier and more logical than in the past. This is a major step forward in helping reduce exacerbations, hospitalizations and death related to asthma, and in limiting side-effects due to standard-of-care medications. Biologics – which hold promise in delivering clinical remission in asthma – are the subject of many ongoing research projects. Newer therapies have a particular role in more severe disease, in the later GINA steps, which also use increasing doses of the ICS-formoterol combination. The pressure is on for the US to mirror initiatives in the UK and other European countries to provide regulatory approval and subsequent insurance coverage for these drugs along with much-needed access to care for patients.
References
1 Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–1222. doi:10.1016/S0140-6736(20)30925-9
2 Mortimer K, Reddel HK, Pitrez PM, Bateman ED. Asthma management in low and middle income countries: case for change. Eur Respir J. 2022 Sep 15;60(3):2103179. doi: 10.1183/13993003.03179-2021. PMID: 35210321; PMCID: PMC9474897.
3 Gaston B, Gardner DD, Mahan K, Akuthota P, Mendonca EA, Durrington H, Marozkina N, Martinez-Nunez RT, Newcomb D, Ainsworth B, Owora AH, Chung KF, Walker S, Fowler SJ, Siddiqui S, Winders T, Zein J, Jarjour N, Huang YJ, Cahill KN, Djukanovic R. Asthma innovations from the first International Collaborative Asthma Network forum. ERJ Open Res. 2023 May 30;9(3):00090-2023. doi: 10.1183/23120541.00090-2023. PMID: 37260461; PMCID: PMC10227632.
4 Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020 May;75(5):1023-1042. doi: 10.1111/all.14221. Epub 2020 Feb 24. PMID: 32034960.
5 Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest 2020; 157: 790–804. doi:10.1016/j.chest.2019.10.053
6 Venkatesan P. 2023 GINA report for asthma. Lancet Respir Med. 2023 Jul;11(7):589. doi: 10.1016/S2213-2600(23)00230-8. Epub 2023 Jun 8. PMID: 37302397.
7 Reddel HK, Bateman ED, Schatz M, Krishnan JA, Cloutier MM. A Practical Guide to Implementing SMART in Asthma Management. J Allergy Clin Immunol Pract. 2022 Jan;10(1S):S31-S38. doi: 10.1016/j.jaip.2021.10.011. Epub 2021 Oct 16. PMID: 34666208.
8 Brackett NF, Pomés A, Chapman MD. New Frontiers: Precise Editing of Allergen Genes Using CRISPR. Front Allergy. 2022 Jan 17;2:821107. doi: 10.3389/falgy.2021.821107. PMID: 35386981; PMCID: PMC8974684.
9 University of California San Francisco [Internet]. UCSF; 2023Apr17 [cited 2024Feb15]. Press release. Editing Genes in the Microbiome to Prevent Disease. Available at: https://www.ucsf.edu/news/2023/04/425141/crispring-microbiome-prevent-childhood-asthma
10 Matera MG, Ora J, Calzetta L, Rogliani P, Cazzola M. Investigational anti IL-13 asthma treatments: a 2023 update. Expert Opin Investig Drugs. 2023 May;32(5):373-386. doi: 10.1080/13543784.2023.2215425. Epub 2023 May 18. PMID: 37194672.
11 Global Initiative for Asthma. GINA; 2023Jul12 [cited 2024Feb15]. Web page. Updated: 2023 What’s New in Gina Slide Set. Available at: https://ginasthma.org/wp-content/uploads/2023/08/GINA-2023-Whats-New-Slides-WEBSITE.pptx
12 U.S. Food and Drug Administration [Internet]. FDA; 2022Nov8 [cited 2024Feb15]. Briefing Document. Available from: https://www.fda.gov/media/162912/download
Related solutions
Specialized expertise and customized solutions across 14 therapeutic centers of excellence, including oncology, GI/NASH, pediatrics, neurology and rare diseases.





