R&D pipelines are rich with innovation in areas of high unmet need, driving a surge in promising new treatments for patients with niche and rare diseases. Under pressure to accelerate approval, regulators are increasingly authorizing use on the basis of single-arm efficacy data. Thus, HTA submissions based on single-arm trial data are rapidly increasing and external comparators are becoming essential, but what type of external comparator approach is best? Here, we explore trends in HTA submissions and outcomes that can inform your strategy.
IQVIA’s HTA Accelerator provides instant insights into payer decision-making from HTA reports of more than 100 agencies in 32 countries across all therapeutic areas. Based on our data extraction parameters we’ve identified 433 single-arm clinical trial submissions to HTA bodies, looking back to 2011. Starting from initial submissions in 2011, we see a subsequent 13-fold increase to 2019, mostly in oncology indications (see Figure 1).
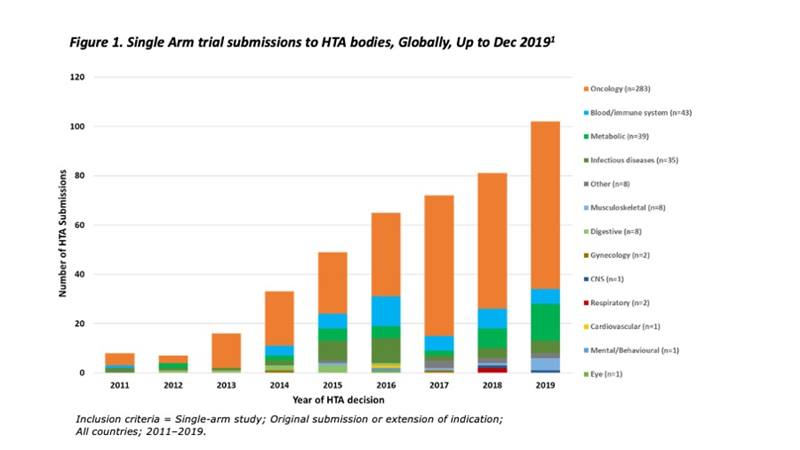
Reproduced from: Patel, D et al. Use of External Comparators for Health Technology Assessment (HTA) Submissions Based on Single-Arm Trials. Value in Health, 2021, In Press.
Trend towards real world data external comparators
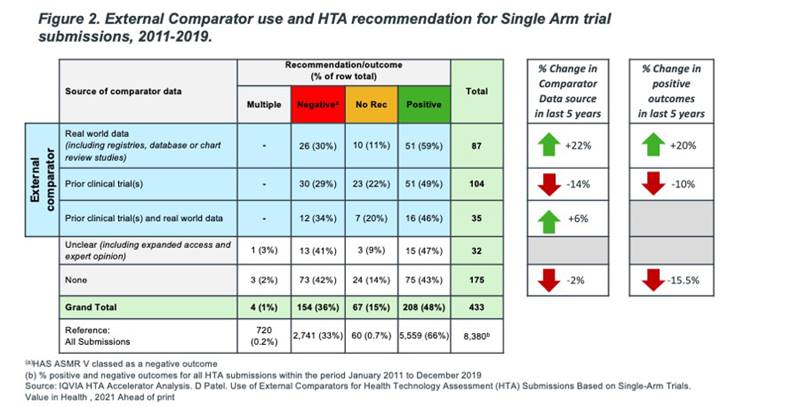
External comparators are rapidly becoming an expected part of non-RCT HTA submissions. Our analysis shows that since 2011, 52% (226/433) of submissions contained some type of external comparator data and the proportion of submissions using no external comparator remains relatively unchanged. However, the type of external comparator datasets used is changing. In the latest 5-year period (2015-19), use of real world data (RWD) external comparators increased by 22%, whereas use of prior trial data as an external comparator decreased by 14%.
Which type of external comparator dataset will most likely lead to a successful HTA outcome? This is hard to say definitively due to the multidimensional nature of HTA decision-making and orphan status, but our analysis shows that there are clear trends in acceptability. Over the last 5 years (Between 2015-17 and 2018-19), the acceptance rate for RWD external comparators increased by 20% while acceptance of prior trial external comparators decreased by 10%, and acceptance of HTA submissions with no external comparator submissions decreased 16%. (see Figure 2).
Which methodological approach for your external comparator study?
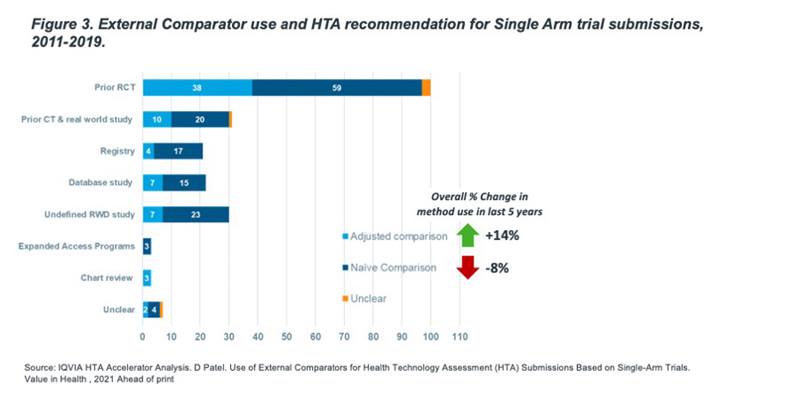
External comparator studies can vary in approach, not only by type of data source but also by method of the comparison. We analyzed whether companies where using a simple, non-statistically adjusted (or ‘naïve’) comparison approach or if statistical adjustment methods were used (either direct propensity score methods or indirect population adjustment methods) (Figure 3).
It is often hard to determine the statistical approach used from HTA appraisals as the level of information varies on these methods. However, from what we could observe, adjusted methods represented only 29% (66) of all external comparator SAT submissions, but has increased by 14% over the last 5 years. 61% (137) submissions were presumed to use ECs in a naïve comparison context. The use of naïve comparisons decreased 8% in the last 5 years.
While this trend may indicate that adjusted methods will be expected for HTA acceptance, we did not see a significant difference in acceptance in our data. We expect this to change as we see more frequent critique of naïve comparison approaches from HTAs.
Planning your external comparator strategy
We see a trend towards better results when using external comparators and particularly RWD external comparators. However, the presence of an external comparator does not guarantee HTA acceptance. Based on our analysis of post-HTA conclusions, acceptability of the data and appropriateness of the analytical method were important considerations, underscoring the multidimensional nature of HTA decision-making. How, then, can you decide which way to go?
A five-step strategic process
Choosing comparator cohorts isn’t easy. Standards of patient care can change over time and vary by country. Careful evaluation of past HTA decisions in your target indication(s) as well as early input from HTA stakeholders are critical. Before designing an external comparator study, we recommend a strategic approach
- Establish the need for an external comparator
- Identify the factors that will drive success
- Determine the right external comparator group
- Collect the external comparator data
- Ensure the most appropriate methodology for comparison to your study data
IQVIA has a dedicated team of experts with significant experience working with manufacturers to define the strategy and methodology for external comparators supporting submissions to regulators and HTA bodies.
About IQVIA’s HTA Accelerator
IQVIAs HTA Accelerator tool contains over 34,000 HTA publications covering 41 countries and 100 HTA bodies published since 2011. Companies can review indication-specific, clinical evidence and comparators, economic analysis, HTA body critique and recommendations. It also features information about the use of RWE, the types of RWE used, the areas supported and the acceptance by HTA bodies. This enables a data-driven approach to your market access strategy and study design.
Source: Patel, D et al. Use of External Comparators for Health Technology Assessment (HTA) Submissions Based on Single-Arm Trials. Value in Health, 2021, In Press.
For further information contact Sam.Foster@iqvia.com or Dony.Patel@iqvia.com
Explore more in this recent ScienceDirect publication: View here.

























