





















- Blogs
- Drive Value and Reduce Study Burden with Innovative, Real World Enriched Study Methods
Real World Evidence (RWE) is steadily becoming a linchpin across the pharmaceutical product lifecycle. With new advances in data collection, quality and validity, there are even more opportunities to leverage RWE as a rich source of decision-making data that can increase value and participation while reducing the costs and burdens of observational studies. That is the foundation of an Enriched Study methodology.
Physician burden is real
There’s debate about how well non-research centric physicians are represented in studies, despite more and more research taking place outside the confines of randomized control trials. In large part, that is because of the time commitment and burden. IQVIA recently completed market research with a panel of 210 physicians across Europe and North America. The goal was to understand their willingness to participate in observational studies, and if time burden or study fees and grants mattered more to them. Our research revealed that 66% of physicians were more willing to take part in observational research if time burden was reduced, and 80% indicated that time was more of a critical determining factor than study fees and grants.
So, how can an Enriched Study approach reduce that burden? To start, Enriched Studies leverage secondary data that already exists in the healthcare ecosystem and can cut down the number of data entry points on a physician’s case report form (CRF). This secondary data can come from electronic medical records, administrative claims, or other real-world sources. Instead of duplicating efforts, physicians save time and can focus on capturing new information.
The proof is in the research
Take figure 1 as an example. Two country-specific CRFs within the same study took an Enriched Study approach while the other did not. The CRFs for the Enriched approach were 21% shorter on average by removing the need to collect diagnosis and medications via long lists. This saves time and reduces physician burden, leading to an average time savings of 30 minutes per patient visit.
Figure 1: Reduction in the number of total CRF pages when using an Enriched Study methodology seen in a global diabetes study
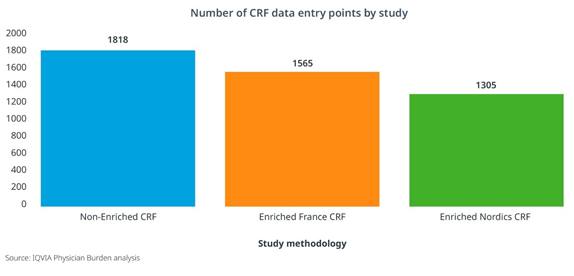
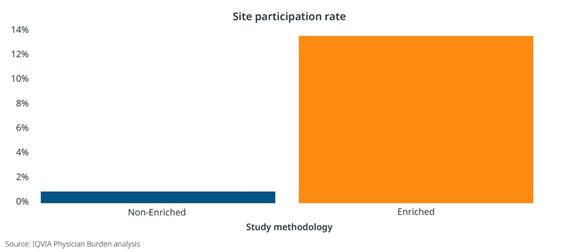
An Enriched Study approach can also positively impact operational costs and even site participation. When the CRF length was shortened as much as 50% through secondary data sources, there was an 8-10% reduction in operational costs related to primary data collection, such as clinical monitoring, data management, and project management. [Figure 2] And in two type 2 diabetes studies with similar target populations and physician profiles, we clearly see increased site participation for the study leveraging an Enriched approach. [Figure 3]
Figure 2: Reduction in clinical monitoring, data management and project management costs relating to shortening of the CRF by 50%
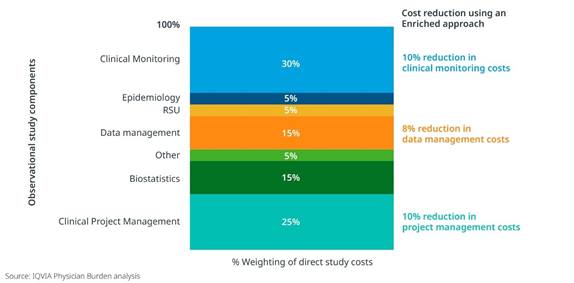
Figure 3: Comparison of site participation using an Enriched Studies methodology to a non-Enriched methodology in two similar phase IV studies involving primary care physicians
Expanding reach of RWE efficiencies
As the data infrastructure grows, and quality and validity increase, more opportunities to leverage RWE to drive efficiencies across different study types are emerging. Reducing the burden of data collection in observational research can benefit both investigators and research nurses in almost all situations. For example, pragmatic trials can use existing data to minimize the impact of data collection during routine care. In some cases, the site may be exempt from completing a CRF entirely if the data ecosystem supports the study variables.
Chances to apply Enriched Study methodologies will continue to expand, establishing the approach as a core innovation to decrease burdens and costs, while increasing value and participation. To learn more about Enriched Study methodologies, click here to listen to our webinar or please contact Joshua.Hiller@iqvia.com for more information.





