IQVIA Medical Research Data (IMRD)
IQVIA Medical Research Data (IMRD)
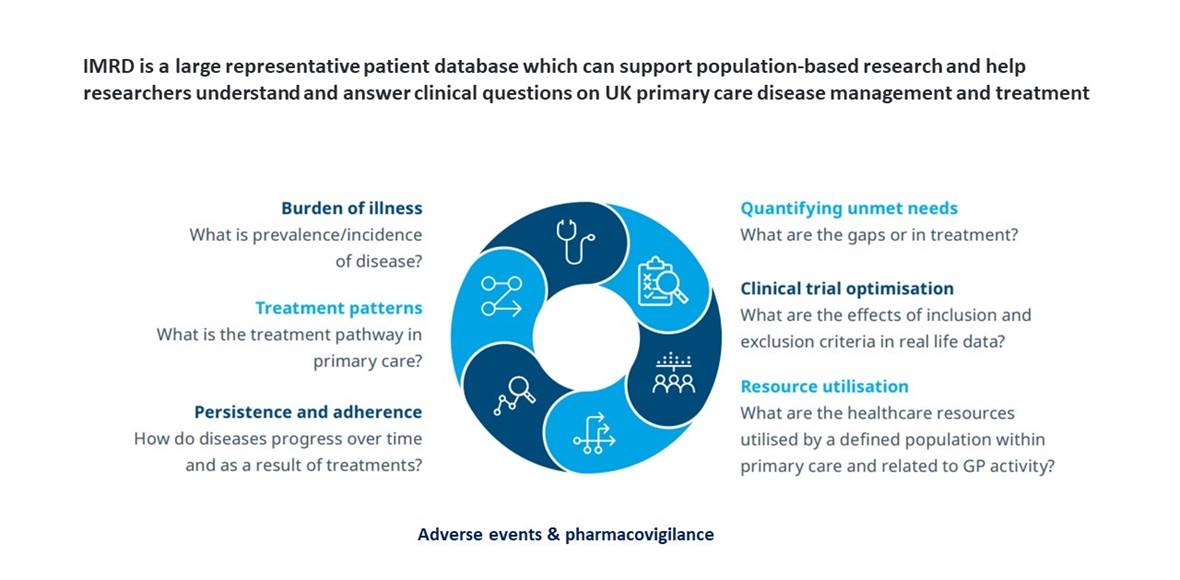
IQVIA Medical Research Data (IMRD) is real world data, which contains the longitudinal non-identified patient electronic medical records (EMR) from 6 million patients. IMRD is used for medical and public health research and treatment analysis.