IQVIA Regulatory Productivity Tools
Boost your regulatory productivity.
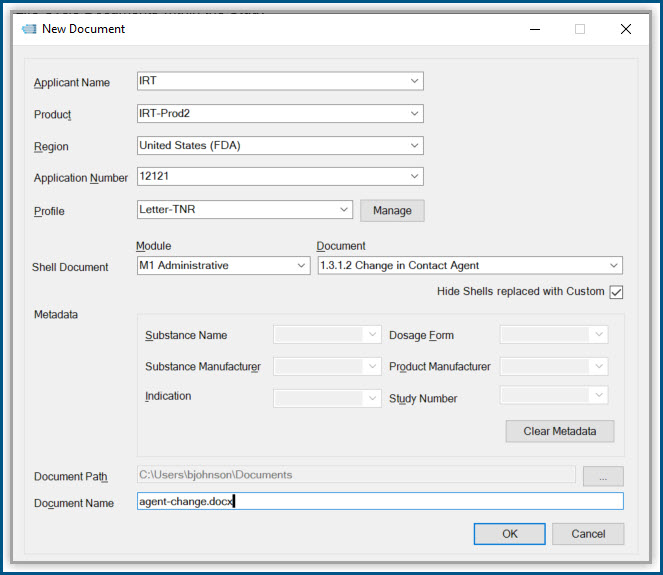
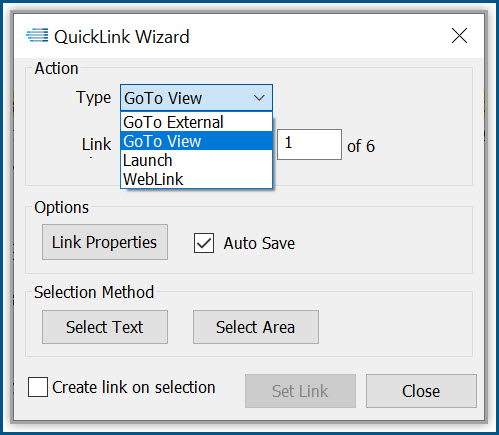
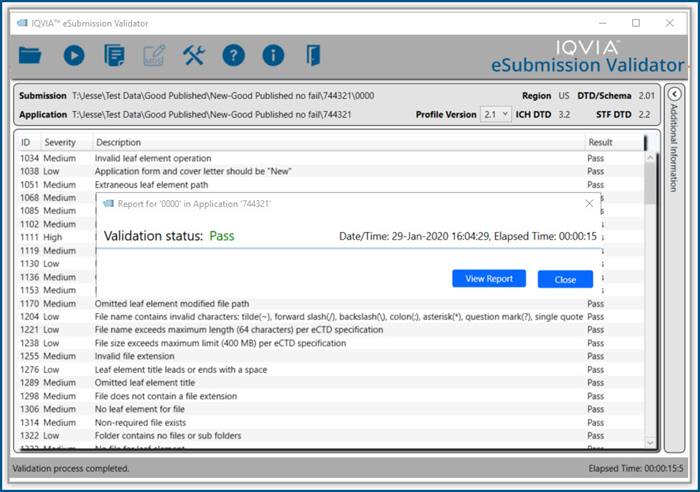
Productivity Tools make it easy to rapidly prepare, publish and validate eCTD and non-eCTD electronic submissions to regulatory authorities, giving your regulatory staff valuable time back for higher-level activities.
Get a 14 Day Free Trial. We'll have you setup within days!