





















About IQVIA Pakistan
About IQVIA Pakistan
IQVIA Pakistan is the first multinational Contract Research Organization (CRO) to establish a dedicated clinical research operations unit in the country. Since 2019, the team has been delivering world-class clinical trial services that meet global standards while leveraging deep local expertise.
IQVIA Pakistan collaborates closely with:
- Tertiary care hospitals
- Clinical trial units
- Key opinion leaders (KOLs) across the country
These partnerships support timely and reliable feasibility assessments, streamlining processes from site selection to initiation.
As a result:
- Average timeline from Site Selection Visit (SSV) to Site Initiation Visit (SIV): 5–6 months
- Average time for regulatory approvals: 4.5 months
Commitment to Quality
To ensure consistent quality delivery, IQVIA conducts quarterly training programmes with participating sites. These sessions:
- Reinforce data quality
- Support regulatory alignment
- Are regularly recognised during sponsor audits
Regulatory Support by DRAP
Approvals and Start-Up Timelines
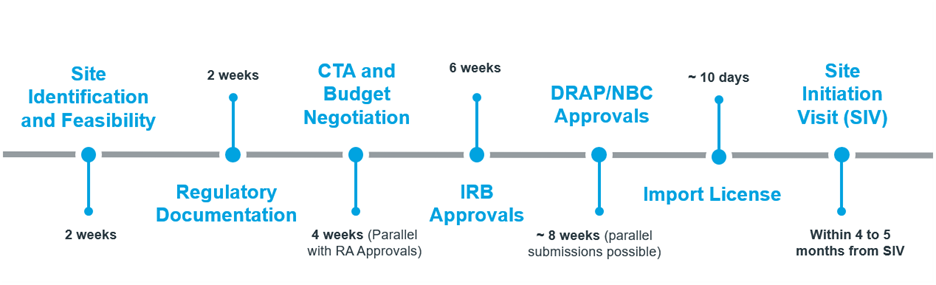
The Drug Regulatory Authority of Pakistan (DRAP) has established a clear and efficient pathway for clinical trials to begin within 3–4 months. Key features include:
- Parallel DRAB and NBC submissions to shorten timelines
- Regulatory reviews typically completed within 6 weeks
- Import licences granted in as few as 10 days
- Mandatory DRAP licensing for CROs and sites, ensuring sponsors work with qualified, GCP-compliant institutions
Compliance and International Standards
DRAP’s oversight is aligned with WHO and ICH guidelines, supporting global best practices. Highlights include:
- Bilingual patient materials required in Urdu and English
- Authorisation of clinical trial units for early-phase and complex studies
- Collaboration with CROs, investigators, and the Ministry of Health to ensure smooth import/export of study materials
- Introduction of digital submission portals to enhance transparency
These measures position Pakistan as a reliable setting for trials in:
- Oncology
- Hepatitis
- Infectious diseases
- Vaccines
Therapeutic Area Expertise
Proven Execution Across Complex Therapeutic Areas in Pakistan
Between 2018 and 2024, IQVIA Pakistan has successfully managed over 24 clinical trials across a wide range of disease areas. These include pivotal studies in Phase II, III, IIIb, and IV programmes, conducted in partnership with multinational sponsors.
Key strengths include:
- Execution of trials in high-burden disease areas
- Time-bound regulatory approvals
- Rapid patient enrolment across diverse sites
This track record demonstrates IQVIA Pakistan’s ability to deliver complex clinical programmes with precision, speed, and regulatory alignment.
|
Therapeutic Area |
Representative Indications |
Phases Covered |
|
Infectious Diseases |
COVID-19, Influenza, Viral Hepatitis C, General Viral Infections |
II, III, IIIb |
|
Gastrointestinal |
Ulcerative Colitis, Malnutrition (Caloric Deficiency) |
II, III |
|
Endocrinology |
Diabetes Mellitus Type 2 |
IV |
|
Oncology |
Breast Cancer |
III |
|
Hem-Oncology |
Myelodysplastic Syndrome |
IV |
|
Hematology |
Paroxysmal Nocturnal Hemoglobinuria (PNH) |
III |
|
Ophthalmology |
Age-related Macular Degeneration |
III |
|
Acute Care |
Pain |
III |
RDS Services in Pakistan
IQVIA Pakistan offers comprehensive CRO services, supporting sponsors across Phase I–IV clinical trials and vaccine research. Our operational capabilities span the full study lifecycle, with a focus on regulatory alignment, site readiness, and data quality.
1. Site Identification & Feasibility
- Access to large treatment-naïve patient populations
- Feasibility assessments across 36 DRAP-approved hospitals
- Site activation within 30 days; up to 13 sites launched in a single month
2. Regulatory & Start-Up
- Parallel submissions to DRAP, NBC, and IRBs where permitted
- Country-specific templates for Clinical Trial Agreements (CTAs) and Delegation Letters
- Import license approvals typically processed within 10 working days
- Patient-facing materials and informed consent forms available in English and Urdu
3. Clinical Operations & Monitoring
- Nationwide CRA coverage across Karachi, Lahore, Islamabad, and Peshawar
- Risk-Based Monitoring (RBM) and traditional on-site monitoring models
- End-to-end study management from start-up through close-out
- Safety oversight and pharmacovigilance support
4. Contracts & Budget Management
- Contract negotiations aligned with DRAP and sponsor requirements
- Transparent budgeting frameworks with local compliance guidance
5. Quality & Compliance
- Dedicated quality resources conducting Focus Site Visits to uphold study integrity
- eTMF integration rate exceeding 95%; CTMS compliance over 97.7%
- Local quality control specialists ensuring audit readiness
6. Flexible Resourcing (FSP Models)
- Dedicated CRAs, CTAs, RSU staff, and QC support available
- Hybrid models supporting both IQVIA-led and sponsor-led studies
Regulatory Timelines with DRAP
Pakistan – The Emerging Clinical Trial Hub
- Pakistan is among the fastest-growing destinations for global clinical trials:
- 200M+ population with large treatment-naïve patient pools
- Fastest recruitment rates worldwide in multi-country programs
- Cost-efficient operations with English-speaking workforce
- Globally recognized infrastructure – JCI/ISO hospitals, WHO-inspected labs
- Strong DRAP collaboration and smooth MoH/site connectivity
- Economical site budget for clinical studies
- Smooth import and export process to manage biological supplies and samples
Therapeutic Leadership Areas in Pakistan
Oncology Trials: Breast, ovarian, lung, ocular, pediatric oncology
Hepatitis Research: HBV, HCV, HDV, with leading liver & GI sites
Infectious Diseases: COVID-19, Influenza, Tuberculosis, Pneumonia
Gastroenterology & MASH Trials: Liver and GI research with global sponsors
Endocrinology & Diabetes: Addressing a burden of 34.5M adults with diabetes





