





















- Blogs
- The impact of the Certificate of Supplementary Protection regulations implementation in Canada
Background
In September 2017, Canada introduced the ‘Certificate of Supplementary Protection’ (CSP) under the Patent Act, upon the recommendation of the Ministry of Industry. The implementation of CSPs allow medicinal products (with first market authorisation) in Canada to potentially extend patent protection for a maximum period of two years. This change to the Canadian patent landscape was required in order to maintain Canada’s commitment with the European Union under the Comprehensive Economic and Trade Agreement.
Patent term extensions (PTEs) have long been established in countries such as the US, Australia, European nations, Japan and South Korea (Table 1). They were designed to allow pharmaceutical companies that have received the first market authorisation, the opportunity to be compensated for their financial investments and time spent on clinical research, in order to obtain market approval. By extending the period of protection on a patent that claims a medicinal ingredient or medicinal combination, generic competitors are impeded from entering the market. The amendments to the Patent Act in Canada allow companies to obtain this form of protection with the Therapeutic Products Directorate (TPD). CSPs can only be applied to a drug product that is market authorised with a Notice of Compliance (NOC) by Health Canada, after the law has come into effect.
| Country | Name | Year Implemented | Period of Protection (Maximum Term) |
| Australia | Patent term extension | 1998 | 5 years |
| Canada | CSP | 2017 | 2 years |
| France | Supplementary Protection Certificate (SPC) | 1992 | 5 years |
| United Kingdom | SPC | 1992 | 5 years |
| Germany | SPC | 1992 | 5 years |
| Japan | Patent term extension | 1988 | 5 years |
| South Korea | Patent term extension | 1987 | 5 years |
| United States | Patent term extension | 1984 | 5 years |
Table 1: Examples of patent term extensions and SPCs tracked in Ark Patent Intelligence
CSP Criteria
The regulations along with the Patents Act outline the amendments, which have been used to establish the CSP criteria. Defined in sections 104-134 of the Patents Act, the Regulations have outlined the specific details of when a CSP can be applied and the scope of coverage. The following summarises a portion of the application criteria:
-
Medicinal ingredients
Only one CSP can be granted per medicinal ingredient or medicinal combination, where the NOC was the first market authorisation for the particular medicinal ingredient(s) in Canada.
If a molecule structure of two medicinal ingredients vary with respect to one another, in that the compound is an ester, salt, complex, chelate, clathrate or non-covalent analogue; the two medicinal ingredients will be considered as the same. Two medicinal products are also considered to be the same if their variation falls into the following categories: enantiomer or a mixture of enantiomers, solvate, polymorph, or an in vivo or in vitro post-translation modification with respect to one another.
Medicinal combinations containing two different medicinal products can also be granted CSPs in Canada. The Patent Act defines that any two combinations that contain the same medicinal ingredients with respect to one another, but differ in dose or strength, are not considered to be different.
-
Patent eligibility
CSP applications that are filed must be placed on a patent that is in force according to subsection 3(1) of the CSP Regulations. The patent itself should claim one or more of the following: the same medicinal ingredient(s), the use of the same medicinal ingredient(s), or the same medicinal ingredient(s) by process of preparation, in which it was market authorised for sale. For patents that claim the ‘use’ of the medicinal ingredient(s), the use specified in the claims does not need to match the approved use when receiving market authorisation, however, the claimed use must set out the use in humans or animals. Applications concerning combinations require all the medicinal ingredients that have been market authorised in the medicinal product to be mentioned in the claims, in order to be eligible. Only one eligible claim is required for the CSP application.
Patent claims that are specific for formulations containing the medicinal ingredient(s) or preparations of a formulation containing the medicinal ingredient(s) will not be considered eligible for CSP applications.
-
Timely submission
The Patent Act outlines that a CSP application must be filed with the TPD prior to, or within a reasonable time period from, when approval was sought for the drug or drug combination in the CSP application.
A CSP application can be filed when:
- A New Drug Submission (NDS) is filed with Health Canada and the medicinal ingredient or medicinal combination is already approved in one of the following countries: the European Union (or EU nations thereof), the US, Australia, Switzerland or Japan (specified in paragraph 6(1)(a) of the CSP Regulations).
- Marketing approval has been submitted in aforementioned countries and within 12 months of when the NDS was filed in Canada.
- NDS submission was within 24 months of marketing approval applications in any of the aforementioned countries, up until the 21st of September 2018 (this is due to a transitional period of the CSP implementation).
Registered CSP
With more and more drug products facing generic competition, the implementation of CSPs into Canada will be beneficial for pharmaceutical companies attempting to maintain market share for longer periods. Canada, however, has only implemented a maximum term of two years for PTE, whereas other countries with established PTE regulations have opted for a maximum term of five years (Table 1).
The CSP period is calculated by subtracting the patent application filing date from the market approval date, and then subtracting a further 5 years. If this period is more than 2 years, the period of protection will be reduced to 2 years.
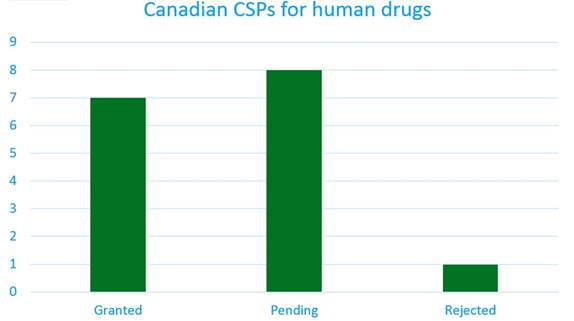
At present, only a small number of CSP applications have been submitted. This information can be found in Ark Patent Intelligence, where the CSP will display the status, subject and calculated extension expiry date.
Summary
With the change in the Canadian Patent Act, any new innovator drugs that are approved for the Canadian market can apply for CSPs, potentially delaying the entry of generic and biosimilar competition for an additional two years. While CSPs cannot be filed for all drug products that are currently market authorised in Canada, there is the potential for some drug products that are in the market to have their period of protection extended if they fall within the criteria of the Patent Act. Generic manufacturers looking at the Canadian market will need to monitor patent term extension expiries, as this could affect the launch of their products.
Ark Patent Intelligence is produced by IQVIA, a global provider of intelligence for the pharmaceutical sector, and provides solutions to pharmaceutical firms in every continent. To find out more, please visit https://www.iqvia.com/our-customers/generics-manufacturers/ark-patent-intelligence





