





















- Blogs
- Pharmacovigilance Automation Has Arrived
SO WHY AREN’T PHARMA COMPANIES LEVERAGING THE POWER OF THESE AI-DRIVEN PLATFORMS?
The rising cost of pharmacovigilance (PV) tasks has been an ongoing challenge for the pharma industry. Many companies have chosen to shift these tasks to overseas vendors to take advantage of cheaper labor without compromising quality. However, as the benefits of outsourcing have increased so too have the costs, forcing companies to shift their operations from country to country in an ongoing quest to find cost-effective labor pools. We’re starting to see this model hit its limits as companies can no longer find enough workers at desired cost levels in these countries to handle the PV workload.
At the same time, the volume of PV cases has steadily risen as regulators push doctors to report more incidences, and patients share their adverse event stories via chat groups, registries and social media. Regulators and industry bodies are also seeking more in-depth trend data that looks beyond individual cases to identify signal trend patterns across treatment categories and patient populations. They want to see companies increasing their use of AE reports to gain greater insights about safety and support future decision making.
The combined pressures of cost, volume and heightened demand for analytics are forcing the industry to embrace advanced technology to support their PV activities.
From Data Entry to Data Analysis
When pharma companies implement advanced PV platforms, they can transform their entire pharmacovigilance workflow. The benefits start with automated reporting, which reduces the time and labor costs, while speeding the delivery of information.
These platforms can also generate more value from PV tasks than was previously possible. Platforms featuring intelligent automation and predictive analytics capture and translate adverse event data from multiple channels and conduct deep analyses of integrated data sets to identify meaningful safety trends. This helps PV professionals better predict and prevent future adverse events and better understand safety issues and related opportunities across the treatment landscape. These added benefits result in reduced errors related to manual data entry and strict patient data privacy controls, minimizing pharmacovigilance-related risks to the business.
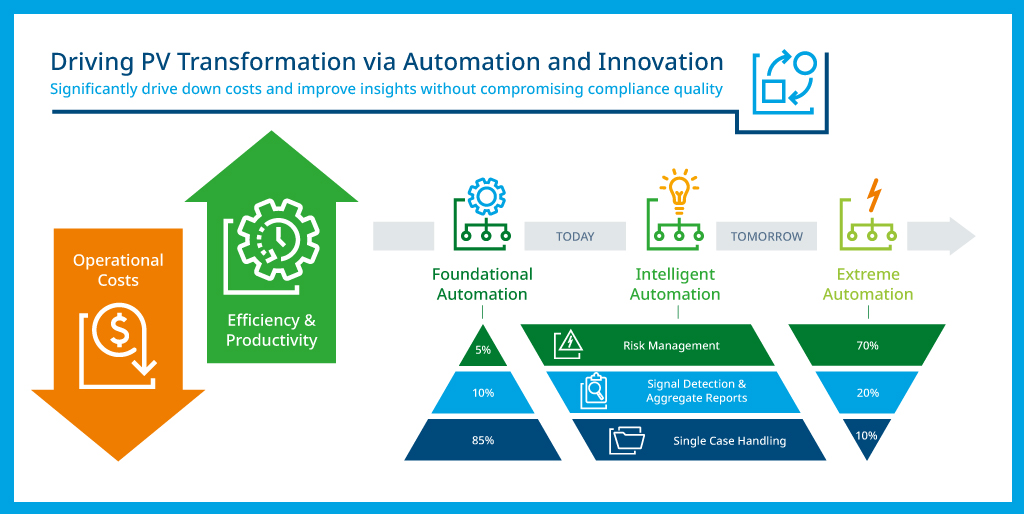
The current generation of pharmacovigilance platforms offers a variety of automation features and can have automation added including:
- Automated intake via web and mobile apps, with results sent directly to the safety system
- Adverse event tracking tools that can analyze structured and unstructured data in real time from multiple channels, including social media and chat bots
- Intake bots that can capture information from PDFs, XML, and other document formats, with translation into PV case submissions
- PV query tools that complete report and case management documentation
- Natural language processing tools capable of analyzing sophisticated narratives including patient charts, social media posts, articles, and other unstructured data
- Analytics tools that can identify safety trends across populations to educate healthcare professionals, regulators and payers
When pharmacovigilance technology fails
This is where many companies stumble. Whether they opt to build a technology platform in-house, or work with a vendor to replace their formerly manual and/or outsourced pharmacovigilance tasks, these projects face a high a risk of failure for a number of reasons.
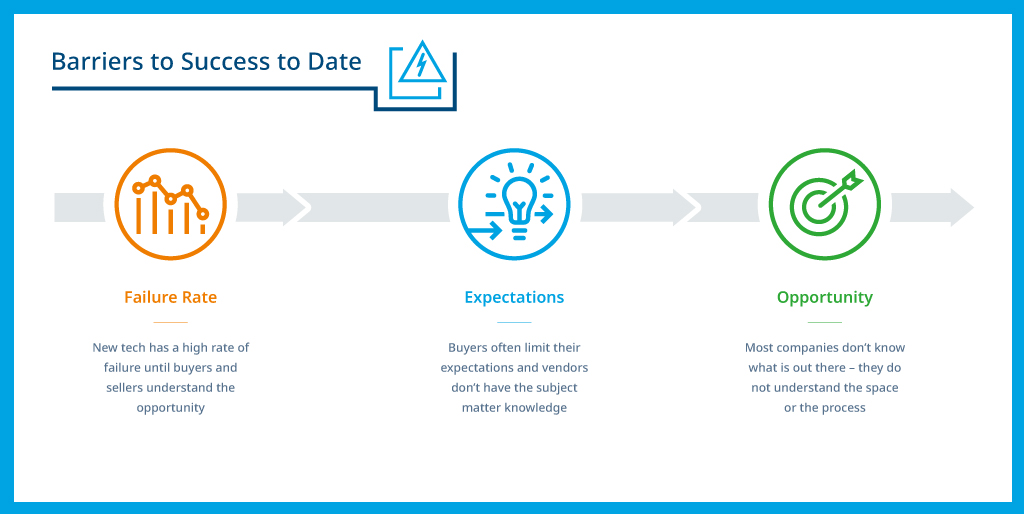
- Uncertainty: Many pharma organizations don’t fully understand the potential that automated PV reporting platforms can bring to their processes, making it difficult for them to devise comprehensive specifications for their own developers, or to adequately vet vendors and platforms in the market.
- Limited ambition: The lack of knowledge of full platform capabilities may cause them to limit their expectations and choose/design solutions that conform to pre-existing processes rather than seek opportunities to innovate. While this may deliver incremental benefits, it can prevent them from finding a solution that will dramatically reduce the time and cost of their PV operations and improve the quality of the output.
- Lack of industry expertise: Companies may seek out technology experts rather than industry experts, only to discover that these partners have merely adapted an existing platform for pharmacovigilance applications, but they don’t have the expertise to navigate the highly complex rules and regulations that govern global PV practices.
Companies can avoid these risks by working with industry experts who can integrate the best of data analytics tools with the human science expertise to make meaningful data-driven decisions about product safety.
These technologies are already transforming the way pharmacovigilance tasks are performed, and every generation of tools will get smarter and more adaptive, extending applications to solve new pharma challenges in different ways. However, investments in these platforms won’t deliver the desired value until buyers understand their full potential, and partner with experts who have both the deep scientific and technological expertise to deliver innovative solutions that optimize pharmacovigilance activities for better, faster results.
IQVIA Safety, Regulatory, Quality and Commercial Compliance can help you stay ahead of change and focus on what matters. Click here to learn more.





