


























- Locations
- Middle East & Africa
- Getting Quality Medicines to Patients Faster in Africa: How to Solve Access Issues
Africa houses 1.2 billion people, about 11% of the global population. Along with this population burden, the continent also bears a disproportionate burden of disease, with 60% of people with HIV/AIDS living in Africa, and more than 90% of the annual global malaria cases being in Africa1. The continent is also afflicted by significant infectious diseases and increasing non-communicable diseases (NCDs). Africa has faced significant public health challenges due to insufficient access to quality, safe, efficacious, and affordable medical products over the years.2 In 2022, some children reportedly lost their lives from acute kidney injury in Gambia after ingesting imported adulterated cough syrups. Events such as these are not just shocking, but also distressing knowing they could have been easily avoided.
Why is it hard for Africa to have a timely access to quality medicines?
Weak regulatory mechanisms and limited availability of financial and technology support across several African countries limits timely access to quality medicines; details can be found below:
- Weak and fragmented regulatory system: Several African countries have unclear policies and incomplete or incoherent legal and regulatory frameworks. A 2012 study found that it took 4 to 7 years on average to register a new product in Sub-Saharan Africa, compared to just 6 to 12 months in high-income regions3. Furthermore, there is a shortage of competent regulatory professionals along with an underdeveloped regulatory infrastructure. Ineffective regional collaborations exist among the National Medicine Regulatory Authorities (NMRA).
- Presence of a few local and regional pharma manufacturing companies: The continent has approximately 375 drug makers serving over 1.1 billion people, and these companies are mostly concentrated in North Africa. Those in Sub-Saharan Africa are clustered in 9 of the 46 countries. When one compares this figure to 10,500 drug manufacturers in India and 5,000 in China for 1.4 billion population each, then the difference becomes quite apparent4.
- Limited financial and technology support: Most of the African countries operate on limited social benefits or universal health coverage. Additionally, there is a lack of state-of-art healthcare provisions capable of handling innovative global health products. There exists a vast digital divide among African health systems. In most countries, poor linkages are seen amongst different information systems and solutions as they are not designed to capture robust data across multiple sectors.
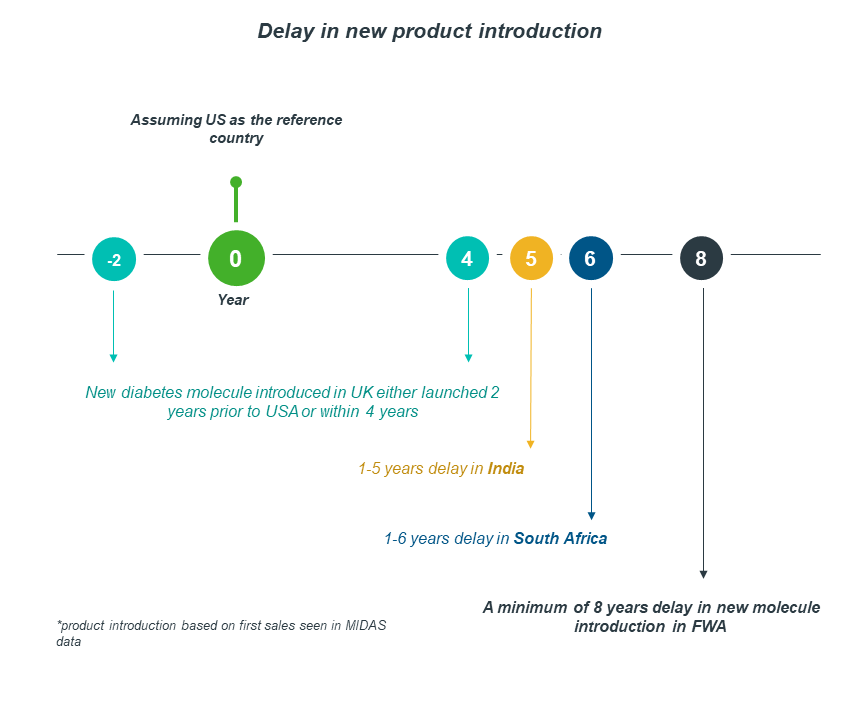
Case-in-point: Significant delays in access to new molecule introductions
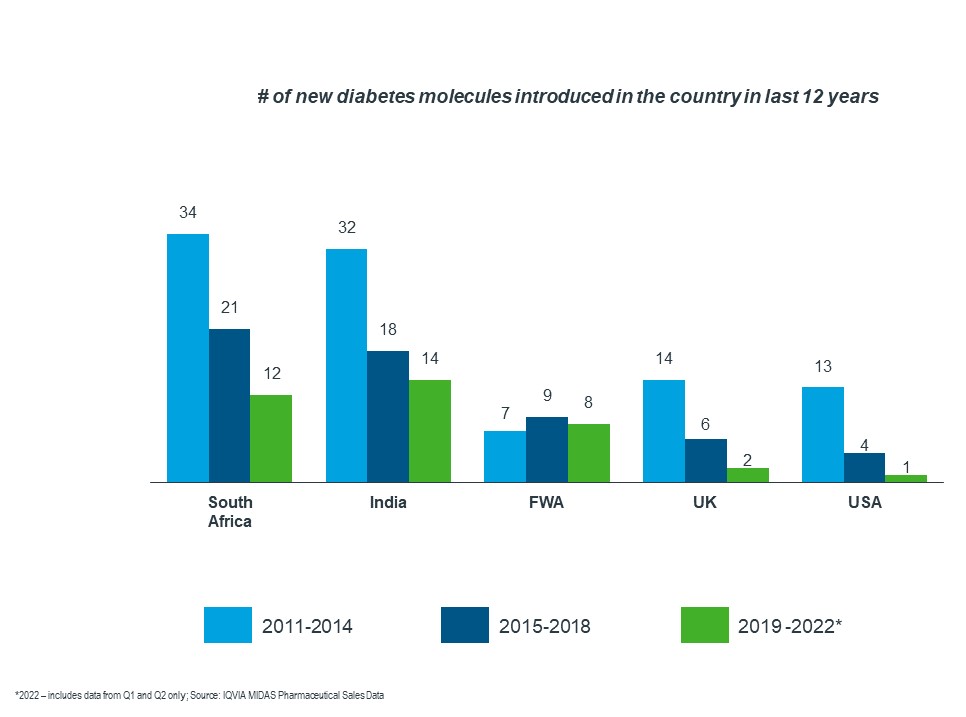
Francophone West Africa and South Africa data demonstrate that, although they have achieved access to quality diabetes medicines, the access is significantly delayed when compared to other nations.
As the graph below shows, there has been a decline in the number of new molecules introduced in various countries. Francophone West Africa had a lower number of new products introduced over the last 12 years when compared to South Africa and India.
Taking the US as a reference country, South Africa faces 1-6 years of delay in product introduction, whereas FWA experiences a minimum of 8 years.
Africa is taking the right steps to tackle the inefficiencies and improve access to medicines
Acknowledging the delays, barriers and hindrances to healthcare product accessibility, Africa has initiated developments to change the regulatory system gaps and inefficiencies to improve medicinal access, as evidenced by the following positive developments:
- Establishment of African Medicine Agency (AMA): Recently, 15 African Union (AU) member states ratified the treaty to create AMA – a continent-wide regulator to complement national and regional efforts and optimize regulatory processes on the continent. It is predicted, that rather than having to negotiate 54 times for each African country, drug and medical device companies would be able to deal with one assessment process and get one recommendation that could be used by all African countries to authorize the product nationally, thereby significantly reducing the product/drug introduction time.
- Introduction of African Pharmaceutical Technology Foundation: The African Development Bank has developed the African Pharmaceutical Technology Foundation (APTF) to boost access to technology in manufacturing medicines and vaccines. So far, African pharmaceutical companies do not have the scouting and negotiation capacity, and bandwidth to engage with global pharmaceutical companies and thereby have been marginalized and left behind in complex global pharmaceutical innovations. The African Pharmaceutical Technology Foundation aims to fill this important gap. However, it will be some time before APTF is fully staffed and completely functional.
What more can be done?
Africa is diverse and comprises of many dissimilar countries with different cultures, market characteristics and economic conditions. While there is no single key to accelerating regulatory and access to medicine timelines, tackling common barriers at country level is a good place to start.
IQVIA’s 2022 whitepaper on Assessment of Access-to-Medicine Timelines in Selected Countries in Middle East and Africa highlights key reforms to improve regulatory and access-to-medicines timelines. These include:
- Holistic and comprehensive regulatory and reimbursement policies/guidelines, in line with the best practices followed by developed nations that are required. Active and passive surveillance of drugs in circulation should be enhanced to keep a check on drug quality.
- Execution of emergency-use approvals and fast-track review are the need of the hour to improve accessibility. Many countries under scope have shown sustained performance in regulatory and access-to-medicine timelines by issuing fast-track registration procedures for new drugs that are already approved by regulators in developed countries such as the US and/or Europe.
- Formal assessment of pharmacoeconomic or cost-effectiveness data plays a limited role in current reimbursement decisions in the MEA region. Several countries in the region have realized a need to strengthen their HTA capabilities to enable local decision makers make sound decisions. For instance, Ghana has a working group reviewing Health Technology Assessments (HTAs) - a defined framework for presenting health economic evaluations.
Ghana has made great strides in its HTA journey. The country currently has a working group reviewing HTAs that have been used to approve indications, such as childhood cancer, for national reimbursement. This has led pharma companies in the country to seek to showcase the value of their products to stakeholders with evidence-based assessments.
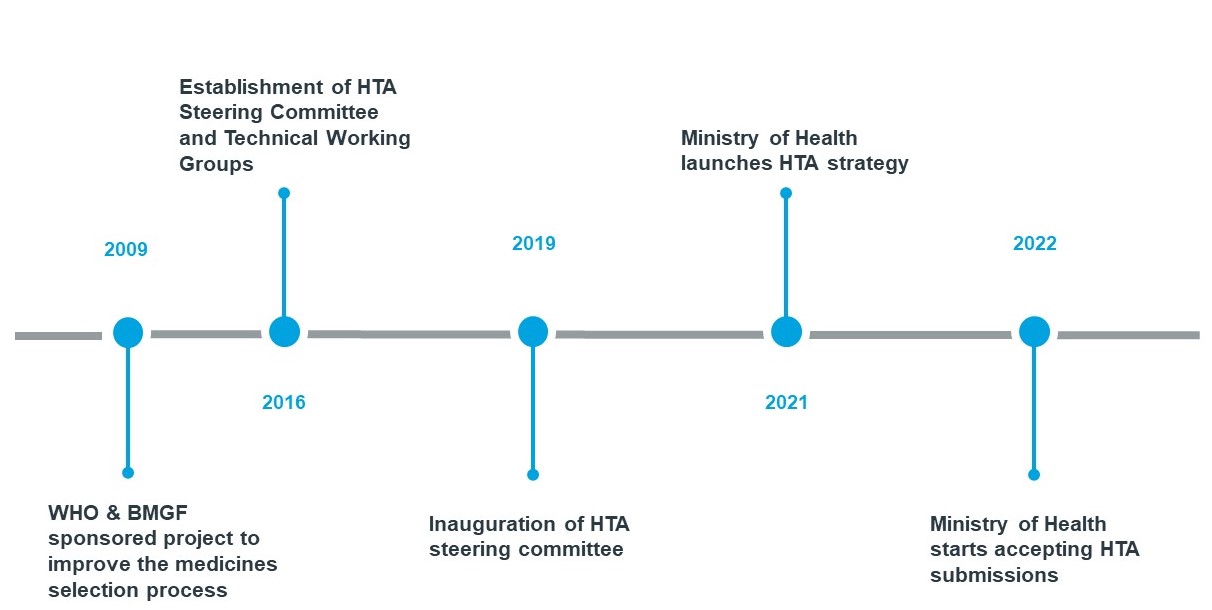
We expect more and more countries in the region will start building their capacity for such technology assessments. In a progressively resource-constrained post-pandemic world, we expect an increase in the requirement for real-world evidence to optimize health spending decisions and ensuring access to the most critical healthcare products.
IQVIA has been supporting its clients by furnishing unparalleled data assets, sharing in-depth healthcare knowledge, and undertaking advanced analytics to meet specific customer needs. IQVIA has a dedicated public-health practice actively engaged with governments, national/international donors and Multilateral health care organizations and private sector stakeholders to support evidence- based decision making. For more information, please reach out to Deepak Batra or Mridu Bhutani
Sources:
- Establishment of the African Medicines Agency: progress, challenges, and regulatory readiness, Journal of Pharmaceutical Policy, and Practice, 2021
- Prevalence and Estimated Economic Burden of Substandard and Falsified Medicines in Low- and Middle-Income Countries, National Library of Medicine, 2018
- Africa’s health security requires strong African regulators, the Africa Report, 2022
- Africa’s Shot at Local Pharma Production, IFC, 2021
- How clinical development can tackle Africa's unique health needs, World Economic Forum, 2022
- What do we need to know? Data sources to support evidence-based decisions using health technology assessment in Ghana, National Library of Medicine, 2020