Investigator Site Portal
Become a Sponsor of Choice with IQVIA Investigator Site Portal
Experience the difference of a complete study platform that sites are eager to use.






















Experience the difference of a complete study platform that sites are eager to use.



The documents, the training, the certificates, the safety letters, having everything in one place makes it so much easier. Honestly, it's been so easy to work with DrugDev Spark [IQVIA Investigator Site Portal]. You know exactly what you're looking for. It's in the same spot every time.

Stacey Klump, AAS, ACRP-CP
Research Operations Coordinator
Clearwater Cardiovascular Consultants
See why so many companies have standardized their clinical operations on the purpose-built, proven platform that gives sites the power to succeed and sponsors the insights they need to improve performance and compliance.

Principal investigators (PIs) engage in clinical research to help their patients survive and thrive with the most advanced science and treatments. But PIs and their staff can quickly become overburdened with paperwork.
Ironically, tech solutions imposed by sponsors often make matters worse, taking attention away from patients:
Identify, select, and activate sites faster with simplified processes, data re-use, and complete oversight for study teams.
Give sites self-service access to all the information they need to be successful.
Quickly and easily push trainings and track compliance to reduce time and cost while increasing audit transparency.
Improve enrollment, retention, protocol adherence, and data quality.
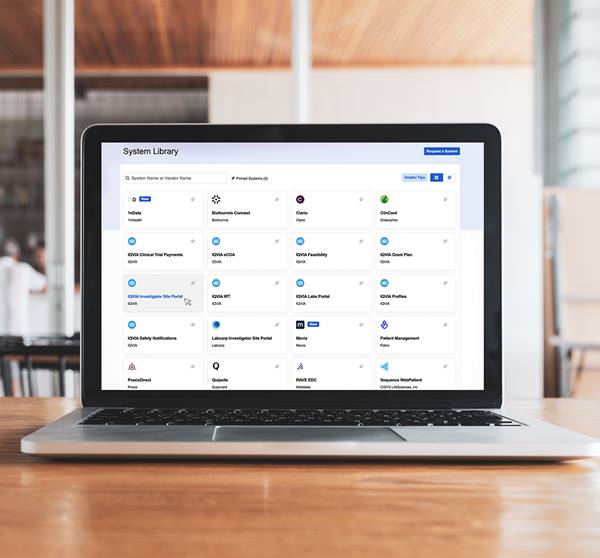
Start, conduct, and complete all your trials more effectively with IQVIA Technologies’ Investigator Site Portal, a collaborative platform designed to make life easier for sites and improve data quality. The Investigator Site Portal is composed of five modules and delivered in either a functional service provider (FSP) or software-as-a-service (SaaS) model.


The IQVIA Investigator Site Portal so far seems to be best in class from the other vendors I have worked with. It's easy to use and navigate and has many features which makes it adaptable for our project and external partners. Highly satisfied with the project management…
Global Study Manager, Top 5 Pharmaceutical Company
2021 IQVIA Technologies Customer Success Survey
Learn ways to speed up site activation and provide a consistent site experience
SaaS-Based Feasibility and Site Selection for Clinical Trials
Reduce cycle time, speed site activation, and keep sites engaged with robust workflows and compliant eTMF filings
Global Pharma Company Dramatically Improves Collaboration, Communication, and Compliance in Trials.
Six-year partnership plays pivotal role in rare disease trials
Communicate SAEs, SUSARs, and other significant events to sites around the world with the Safety Notifications module of the IQVIA Investigator Site Portal.
Give your sites the courses and credits they need to succeed with the Learning Management module of the Investigator Site Portal.
Reduce the high cost of administering site payments and give sites confidence.
Manage trial master files from planning through archive with solutions designed to improve workflow efficiency, promote collaboration, and support compliance.