Harness the power of automation to execute streamlined end-to-end safety solutions while reducing costs.






















- Blogs
- Transform Your Approach to Compliance
From concept to market
Quality, regulatory, safety, and commercial compliance are the building blocks that ensure medicines and devices are safe and effective and that companies operate in an ethical manner. However, the shifting Global Regulatory compliance environment means the impact of these requirements continues to expand, placing an increasing burden on drug and medical device manufacturers. A recent global survey of pharmaceutical CEOs1 shows an increase in regulatory scrutiny across many aspects of the business, including sales and marketing practices, government drug price reporting, data privacy management, and clinical operations. Changes in industry regulations are now viewed as one of the top three disruptive business trends faced by pharmaceutical and life sciences organizations, and many pharma leaders view these changes as a threat to their growth.2
But they don’t have to be.
Compliance has long been viewed as a cost-center and a necessity, but leading organizations are recognizing opportunities for innovation in this space.
Meeting regulatory compliance requirements as efficiently as possible means creating a culture of proactivity to ensure products meet the needs of patients and that the company operates ethically, which translates into company growth, reduced risk, and a stronger brand image in the eyes of the public. Conversely, when companies treat compliance as a must-do rather than a value-add and fail to invest in these processes, they increase risks that could involve compromised patient safety, delays or denials of product approvals, license withdrawals, financial penalties, and in the worst case, criminal penalties. These adverse circumstances can be devastating to the individual life sciences company and generate negative public perception that affects the entire industry.
To avoid these risks and add value to the compliance process, companies need to look for synergies in their regulatory compliance activities that be used to build a business case for technology and talent investment, and to identify opportunities to remove waste and redundancy across the full product life cycle.
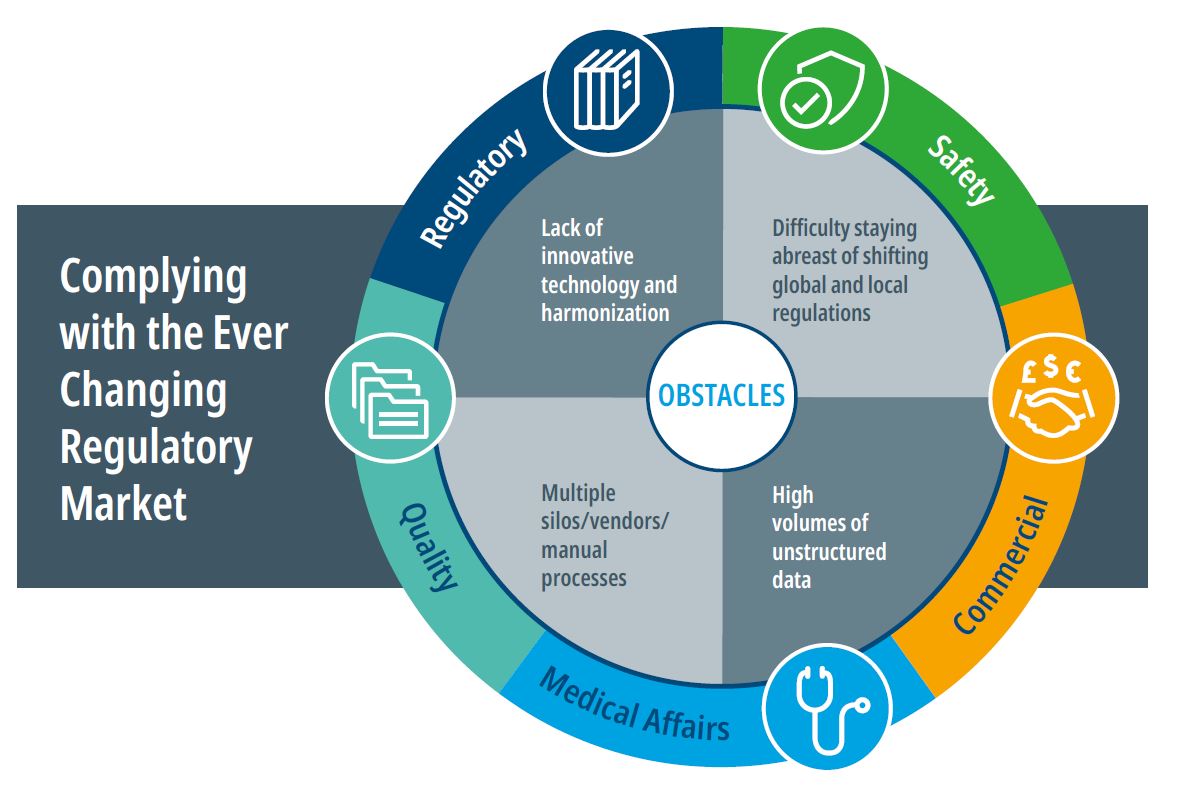
Tearing down obstacles
When companies take a holistic view of the impact of regulations on quality, regulatory, safety, and commercial compliance requirements, they can identify common challenges that restrict overall productivity, and generate hidden waste and risk. This is the first step in driving innovation in the regulatory and compliance environment.
These obstacles typically include:
- Lack of innovative technology and harmonization: While the pharma industry has invested billions in advanced technology, those investments have been slow to trickle down to compliance. Aging and disconnected platforms and lack of automation add time, cost, and poor visibility to the compliance process.
- Difficulty staying abreast of shifting global and local regulations: Global pharmaceutical and medical device companies – and smaller biotech companies with global aspirations – need experts with a keen understanding of global regulations and how they affect the development process. Staying abreast of regulatory changes, by country and region, can be a tremendous resource drain but it vital to achieving and maintaining compliance.
- Multiple silos/vendors/manual processes: Many companies lack horizontal integration of compliance tasks. Numerous vendors, disparate platforms, and lack of automation result in duplicative spending, lack of internal cost transparency, and inefficient operations.
- High volumes of unstructured data. The lack of conformity across data sets complicates downstream reporting and can delay the real-time insights required to maintain compliance.
Once these obstacles are identified, business leaders can make the case for upgrades and investments. These may include implementation of centralized technology systems with advanced analytics capabilities to manage compliance tasks and capture data in a single data repository; hiring new talent or collaborating with industry partners to ensure all development decisions align with the latest global regulatory trends; and harmonizing delivery of external compliance tasks under a single industry partner who can provide deep domain expertise to increase transparency, avoid overlap, and leverage best practices to more efficiently meet all of the organization’s compliance needs.
Taking on the Future
Transforming a company’s approach to compliance takes time and resources. Some may start with pilot projects to update a single piece of technology or to automate one aspect of their reporting, while others will invest in end-to-end solutions that radically change the culture of compliance in their organizations. Regardless of the approach, these companies should do their research, and work with partners who can help them identify near-term projects that will drive early measurable savings and lay the groundwork for larger transformation efforts.
Although these transformations take time, they can significantly lower total costs while enhancing regulatory compliance and, in turn, reducing risks for patients, product development, and company reputation.
---------------------------------------
1 https://www.pwc.com/il/en/pharmaceuticals/managing-regulatory-compliance.html
2https://www.pwc.com/gx/en/ceo-survey/industry/pdfs/pwc-ceo-survey-pharma-life-sciences.pdf

Take a Proactive Approach to Regulatory Compliance
Related solutions
Meet the challenge of changing stakeholder demands and increasing cost constraints with IQVIA's integrated technology services and analytics-driven offerings.





