See how we partner with organizations across the healthcare ecosystem, from emerging biotechnology and large pharmaceutical, to medical technology, consumer health, and more, to drive human health forward.






















Introduction
The impact of antimicrobial resistance (AMR) on healthcare systems is hard to overstate. AMR is now the leading cause of death from communicable diseases, surpassing tuberculosis, and HIV/AIDS and malaria, combined1. In 2019, AMR was directly responsible for 1.25 million deaths and approximately 1.75 million disability-adjusted life years lost, with six bacterial pathogens accounting for over 70% of AMR-related deaths1-3. If this threat is left unchecked, the number of attributable deaths could reach 10 million deaths by 20503. The challenge is truly global, affecting high-, middle- and low-income countries, and may have been exacerbated by the COVID-19 pandemic - in November 2023, for example, the UK’s Health Security Agency showed that deaths due to severe antibiotic resistant infections are now on the rise in England, and antibiotic use, which had declined from 2014 to 2020, has now started to rise once more4.
The urgency to address AMR is therefore more critical than ever. 2024 will be a pivotal policy year for AMR and pandemic prevention, with the United Nations General Assembly High-Level Meeting on AMR and the UN’s pandemic preparedness treaty due to be considered at the 77th World Health Assembly. Key international agreements and policy steps will define the global response to this threat.
Re-starting the innovation engine for antibiotics
Since 2017, there have been 12 antibiotic launches, with an average revenue of US$5 million one-year post-launch. The lack of major commercial success for recently launched antibiotics is a significant challenge for incentivising pipeline investment, meaning commercial incentive alone cannot be relied upon. The commercial reward for today’s top antibiotics is put into stark perspective when considered against historical antibiotic sales, as shown in figure 1.
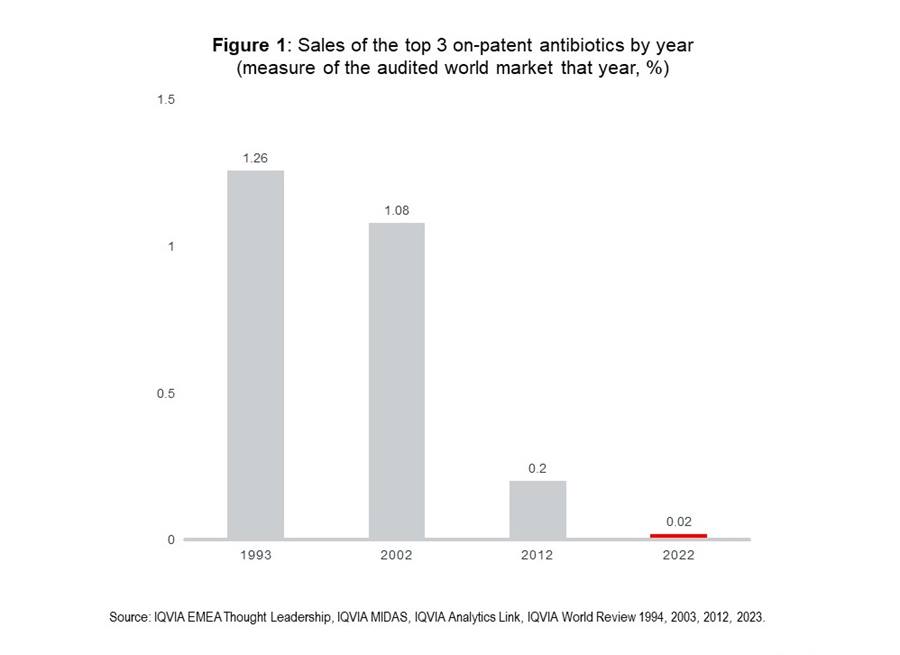
Two to three decades ago, leading antibiotics accounted for a significant share of the global medicines market, over 1% of total global sales value for the three leading antibiotics. This had collapsed by 2012 and is negligible by 2022. This disparity is further highlighted by the fact that zero of the top 300 pharma products by global revenues in 2022 were antibiotics. Volume of antibiotic consumption remains high, but value from commercial sales is extremely low. Further challenge comes from the rise of generics and high cost of antibiotic development, estimated by some to be up-to US$1.5 billion5. Due to such costs, if substantial commercial success is not possible, significant alternative incentives are necessary.
Current policies to incentivise the development of new antibiotics have proven to be regional and, so far, ineffectual. 71% of reward for development are push incentives, highlighting – as a combination of push and pull incentives are key – that revision is required6.
The current antibiotic pipeline: too little, too late?
Maintaining and improving our sparse reservoir of antibacterial agents, especially those active against multi-drug resistant (MDR) pathogens, such as those listed as a WHO priority, is crucial. The pipeline reflects a shift toward targeting resistant pathogens, with 61% of products second-last line treatments, with drug regimens of which typically being longer and more expensive than first-line antibiotics. The size of the pipeline must improve to meet current and future demand, considering that innovation is undermined by rapid resistance emergence, within two-to-three years post-market entry7. This is not a general challenge of development of agents to fight infectious diseases: 106 antibacterial products currently in development are dwarfed by the over five-fold greater number of antiviral products.
The extensive and expensive clinical trial and approval progress, which, from preclinical through clinical stages can be 10 to 15 years, is when most financial, regulatory and scientific challenges are faced by antibiotic developers7,8. Developing products against resistant pathogens requires patients with such infections for trials and can be limited by a small population with such conditions. There would be potential to incentivise development by providing frameworks to reduce the cost, and thereby risk, of antibiotic clinical trials. In excess of 98% of microorganisms, and therefore the antibiotics they synthesise, cannot be cultivated under laboratory conditions. Innovative solutions to improve this outlook could be key in accessing chemical novelty. The significance of novelty is reflected in the current pipeline, shown in figure 2, as a mere 5% of products comprise a novel mechanism of action.
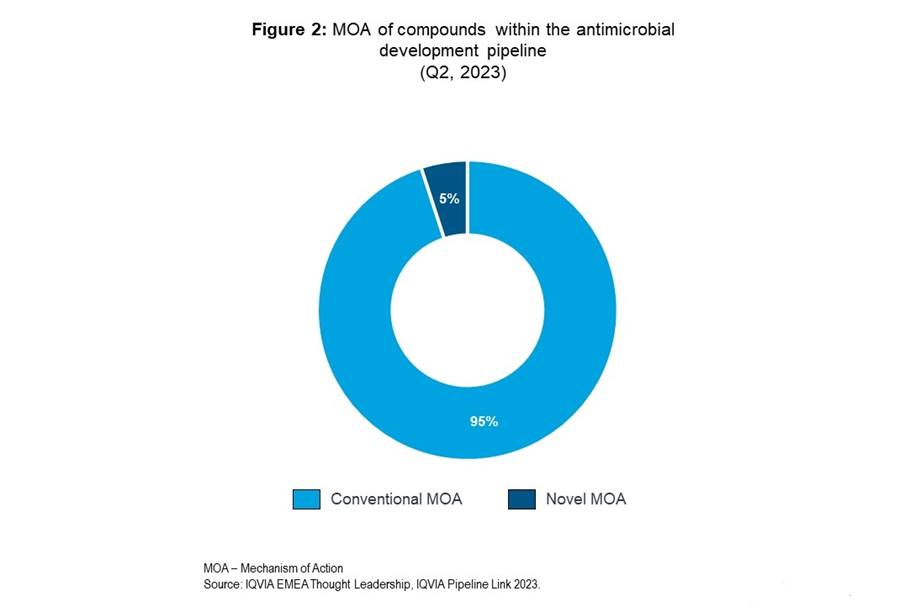
Innovative alternatives to antibiotics are being explored, which could serve as key adjuvants for antimicrobial chemotherapy to provide novel targets. Bacteriophages, for instance, are being revisited as potential solutions, of which there are currently two bacteriophage products in the pipeline, both from Johnson & Johnson.
A holistic approach is required to incentivise innovation
AMR presents a paradoxical situation, with a very salient tension between access and excess. Even as the threat of AMR grows, people also die from a lack of access to the right antibiotics as well as from resistant pathogens. A dichotomy for pharmaceutical companies and healthcare providers will be balancing the development of effective antibiotics and ensuring equitable distribution.
Comprehensive changes across the antibiotic value chain, outlined in figure 3, are imperative in the fight against AMR. A critical issue is the lack of data on antibiotic consumption, particularly across sub-Saharan Africa, which is also grappling with some of the highest AMR-related burden and challenges3. This highlights an unmet need to enhance surveillance infrastructure and diversify data gathering, which, in turn, can integrate real-world evidence into healthcare decisions.
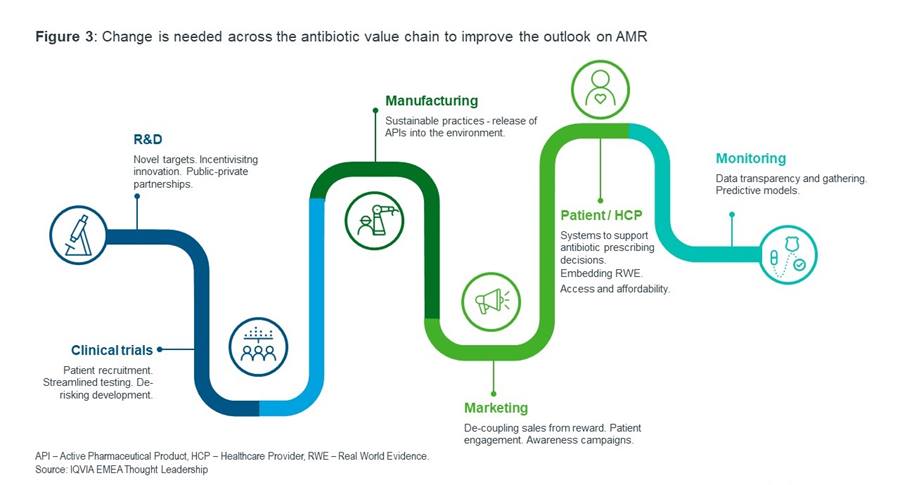
Pull incentives, such as de-linked market entry rewards and an opt-in subscription model – for example, fixed payments for antimicrobial product supply de-linked from volume sold – present as some of the possible solutions to incentivise pharmaceutical companies to improve the pipeline ecosystem to maintain innovation long-term9. For the pipeline to be sustainable for the future, commitments must be made, and adhered to, regarding responsible use of antibiotics. AI and machine learning has potential to drive AMR solutions at key stages of the value chain, particularly in the discovery and optimisation of antibiotics in the pipeline and in better antimicrobial stewardship and surveillance.
A holistic and global approach is needed to bolster the pipeline for future antibiotics and improve the business model and incentivise innovation, as a collective effort to turn the tide on AMR. The stakes could not be higher. Should the situation not improve or worsen, we risk entering a post-antibiotic era, wherein common and minor infections could again become deadly, and MDR superbugs could decimate public health. Lessons from the COVID-19 pandemic, where effective surveillance, use of data and analytics to support effective decision making, and public-private partnerships were deployed to develop innovation, must be applied, especially as the potential for a new AMR pandemic is higher than ever before.
References
IQVIA MIDAS Quarterly. Q2 2023.
IQVIA Pipeline Link. October 2023.
IQVIA World Review
1The Review on Antimicrobial Resistance. (2014). Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations.
2OECD. (2018). Stemming the Superbug Tide.
3AMR Collaborators. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis.
4UK Health Security Agency. (2023). Antibiotic resistant infections and associated deaths increase.
5Plackett. B. (2020). Why big pharma has abandoned antibiotics.
6Wasan. H. (2023). Landscape of Push Funding in Antibiotic Research.
7WHO. (2022). Lack of innovation set to undermine antibiotic performance and health gains.
8European Commission, European Health and Digital Executive Agency. (2023). Study on bringing AMR medical countermeasures to the market – Final report.
9BGC. (2022). The Case for a Subscription Model to Tackle Antimicrobial Resistance.
Related solutions
Use big data and predictive analytics to improve human health.





