





















- Blogs
- Identifying Paediatric Investigation Plans in the European Union
Introduction
Paediatric medicines are known to be associated with various complexities such as higher rate of side effects, adverse drug reactions (ADRs), efficacy issues, and inappropriate formulations. These difficulties during research and development, in turn, result in off-label paediatric use of drug products in several conditions. The off-label use comes with a higher risk of ADRs, including hospitalisation and death. The Paediatric Regulation came into existence in the European Union (EU) in 2007 to facilitate ethical research, development, and availability of safe, efficacious, and high-quality medicinal products in paediatric patients1.
One of the main pillars of this Regulation is Paediatric Investigation Plan (PIP). A PIP needs to be written and submitted with every new marketing authorisation application (MAA) to obtain necessary data through studies in children, to support the development and authorisation of drug products for paediatric use. This requirement also applies for MAA submitted for a new indication, new pharmaceutical form, or new route of administration for the drug products that are already authorised and protected by patent and supplementary protection certificates (SPCs). However, there are certain exceptions where a PIP is not required, such as drug products like traditional herbal, homeopathic, generic, hybrid, and biosimilar products2.
PIP Assessment
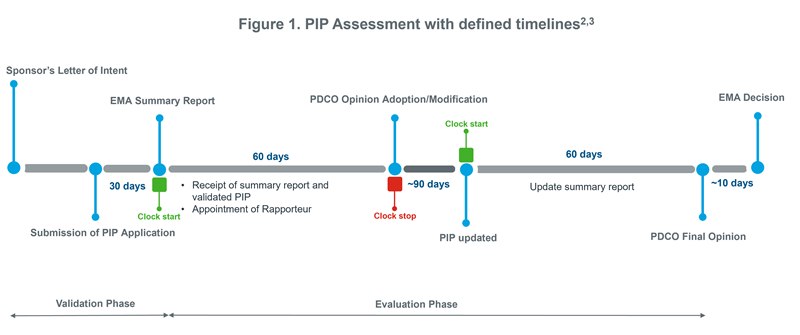
The PIP procedure occurs systematically, in a time-bound manner, and takes about 9-10 months starting from submission to decision. The procedure involves active participation of the sponsor, the European Medicines Agency (EMA) and the Paediatric Committee (PDCO).
As shown in Figure 1, the review procedure occurs in four phases2,3:- Validation: Initially, when the sponsor intends to apply for MAA, a PIP should be submitted to the EMA. Within 30 days of the receipt, EMA shall evaluate the validity of the request, prepare a summary report, and submit it to the PDCO.
- Evaluation: This phase is around 120-days long procedure involving the ‘Clock stop’ and ‘Clock start’. During this, the PDCO appoints a ‘rapporteur’ for the assessment of PIP. The rapporteur presents their findings to the PDCO within 60 days. After this, if the PIP is well-justified, the PDCO will adopt a final opinion, or in case of further modifications, the PDCO may ask questions to the sponsor for additional clarifications or justifications. The sponsor has ~ 90 days to submit its responses, during which the time will be suspended. After the submission, the clock starts again at Day 61, and PDCO has 60 days to evaluate the responses and update the summary report. During the evaluation phase, multiple discussions are held between the sponsor and PDCO at Day 30, 60, 90 and 120.
- PDCO opinion: The PDCO adopts a final opinion at Day 60 or 120. The sponsor may request for re-examination, after which the PDCO adopts a definitive opinion.
- EMA decision: Following the PDCO’s definitive opinion, the EMA takes one of the six decisions within ~10 days. These decisions are PIP agreed (P), PIP refused (RP), modification granted (PM), modification refused (RPM), waiver granted (W) and waiver refused (RW). The decisions ‘P’ and ‘PM’ are referred to as ‘positive decisions’ or ‘positive PIPs’4.
Once the EMA adopts the positive decision, the sponsor may conduct studies as agreed upon in the PIP. The sponsor may request the PDCO to conduct a compliance check ahead of the submission of the MAA. If such a compliance check is not requested, the check is performed as a part of the regular validation procedure of the MAA. During the check, the PDCO verifies whether the studies agreed in the PIP are performed as per the PIP decision and within the agreed timelines5.
In case of a positive decision and positive compliance outcome of the PIPs, the sponsors are granted certain rewards as follows5,6:- For non-orphan drug products, 6 months extension on SPCs is granted.
- For orphan drug products that are awarded 10 years of market exclusivity, an additional two years of market exclusivity is granted.
- Already authorised drug products that are not protected by a patent or SPCs, a paediatric-use marketing authorisation (PUMA) application is to be submitted. If PUMA is granted, the product receives 10 years of market protection.
Along with the rewards, the EMA also provides certain incentives like free scientific advice before the PIP submission or during the implementation and protocol assistance to support the sponsors in developing paediatric drug products5.
Further, sponsors can also request a deferral for delaying the initiation or completion of studies in paediatrics until there is enough data to establish its safety and efficacy in adults. The PDCO may grant deferral if it is well-justified. Thus, deferrals are instrumental in avoiding delays for obtaining marketing authorisation for drug products in the adult population2,7. Also, when a drug product is not likely to be effective or safe in paediatrics or when certain conditions affect only the adult population, a PIP is not required and can be waived off. Specific drug products that do not represent a significant therapeutic efficacy over existing treatments for paediatrics are also exempt from the requirement for a PIP and can also be waived8.
Identifying PIPs
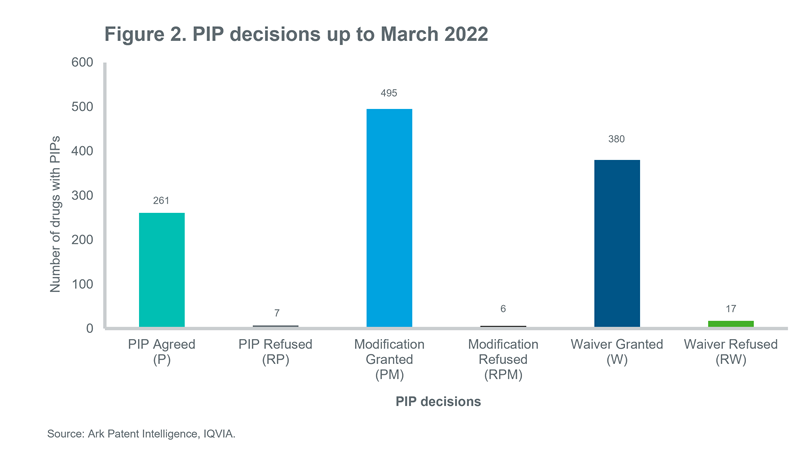
Ark Patent Intelligence shows the most up-to-date PIP data for nearly 1000 drugs as shown in Figure 2. According to the data, the positive decisions, P and PM, account for 65% of the total decisions and waiver accounts for 33% of the PIPs. Further, less than 3% of the total PIP applications were refused.
Conclusion
The inadequate use of off-label drugs in the paediatric population across the EU drives the need for prioritising clinical trials in the paediatric area for developing medicines for specific paediatric indications. The Paediatric Regulation was established in 2007 in the EU to promote the development of safe, efficacious, and quality medicines in the paediatric population. The regulation put forth the mandatory requirement of PIP, the roles and responsibilities of PDCO, various rewards upon completion of paediatric studies, and incentives to support the development of drug products to address the unmet clinical needs in paediatric population. Through the help of other PIP measures such as waivers and deferrals, unwanted studies in the paediatric population can be avoided. Thus, a PIP application helps in providing better medicines, appropriate formulations and reducing off-label use in children.
References
- https://www.ema.europa.eu/en/documents/leaflet/better-medicines-children_en.pdf
- https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32006R1901&from=EN
- https://www.ema.europa.eu/en/documents/presentation/presentation-paediatric-investigation-plan-assessment-procedure_en.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7761547/
- https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/questions-answers-procedure-paediatric-investigation-plan-compliance-verification-european-medicines_en.pdf
- https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/paediatric-investigation-plans
- https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/paediatric-investigation-plans/class-waivers
- https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/rewards-incentives-paediatric-medicines
The data in this article is extracted from IQVIA’s Ark Patent Intelligence Key Patents module.
Ark Patent Intelligence is produced by IQVIA, a global provider of intelligence for the pharmaceutical sector, and provides solutions to pharmaceutical firms in every continent. To find out more, please click here.





