E360 Real World Data Platform
Get Smarter RWD Insights Faster
Leverage E360® a self-service solution that enables you to rapidly access, analyze, visualize, and collaborate on insights from +1 Billion Real World Data global patient records.



Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more

Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more



Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more

Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more
"We strive to help improve outcomes and create a healthier, more sustainable world for people everywhere.
LEARN MORE
Reimagine clinical development by intelligently connecting data, technology, and analytics to optimize your trials. The result? Faster decision making and reduced risk so you can deliver life-changing therapies faster.
Research & Development Overview
Generate and disseminate evidence that answers crucial clinical, regulatory and commercial questions, enabling you to drive smarter decisions and meet your stakeholder needs with confidence.
REAL WORLD EVIDENCE OVERVIEW
Elevate commercial models with precision and speed using AI-driven analytics and technology that illuminate hidden insights in data.
COMMERCIALIZATION OVERVIEW
Orchestrate your success across the complete compliance lifecycle with best-in-class services and solutions for safety, regulatory, quality and medical information.
COMPLIANCE OVERVIEW
When your destination is a healthier world, making intelligent connections between data, technology, and services is your roadmap.
TECHNOLOGIES OVERVIEWExplore our library of insights, thought leadership, and the latest topics & trends in healthcare.
DISCOVER INSIGHTSAn in-depth exploration of the global healthcare ecosystem with timely research, insightful analysis, and scientific expertise.
SEE LATEST REPORTS
By making intelligent connections between your needs, our capabilities, and the healthcare ecosystem, we can help you be more agile, accelerate results, and improve patient outcomes.
LEARN MORE
Building on a rich history of developing AI for healthcare, IQVIA AI connects the right data, technology, and expertise to address the unique needs of healthcare. It's what we call Healthcare-grade AI.
LEARN MORE
The IQVIA Human Data Science Cloud is our unique capability designed to enable healthcare-grade analytics, tools, and data management solutions to deliver fit-for-purpose global data at scale.
LEARN MORE
The IQVIA Innovation Hub connects start-ups with the extensive IQVIA network of assets, resources, clients, and partners. Together, we can help lead the future of healthcare with the extensive IQVIA network of assets, resources, clients, and partners.
LEARN MORE
IQVIA Decentralized Trials deliver purpose-built clinical services and technologies that engage the right patients wherever they are. Our hybrid and fully virtual solutions have been used more than any others.
LEARN MORE
Empowering patients to personalize their healthcare and connecting them to caregivers has the potential to change the care delivery paradigm. IQVIA and Apple are collaborating to bring this exciting future of personalized care directly to devices patients already have and use.
LEARN MOREOur mission is to accelerate innovation for a healthier world. Together, we can solve customer challenges and improve patient lives.
LEARN MORECareers, culture and everything in between. Find out what’s going on right here, right now.
LEARN MORE
"Improving human health requires brave thinkers who are willing to explore new ideas and build on successes. Unleash your potential with us.
SEARCH JOBSLeverage E360® a self-service solution that enables you to rapidly access, analyze, visualize, and collaborate on insights from +1 Billion Real World Data global patient records.



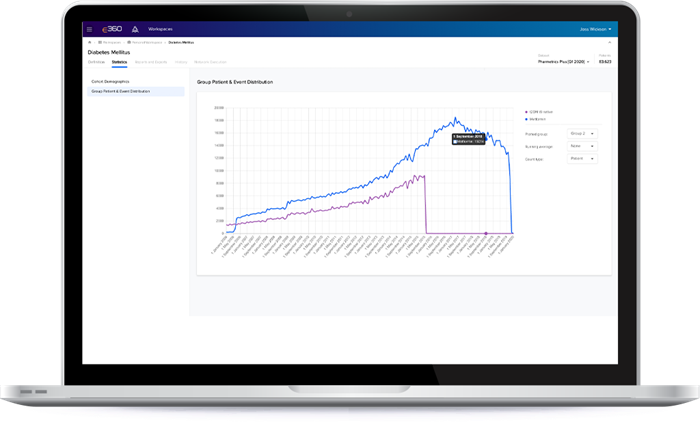
Rapidly solve your most critical business challenges by transforming your approach to asking questions, finding solutions and making decisions. E360® is empowering healthcare stakeholders to ask and iterate multiple questions, quickly get answers and share insights.
You have research questions today; you'll have more next month and still more next year. Move beyond one-off studies to always available insights generation with E360® Self-service Platform, the world’s largest accessible RWD portfolio and Agile Health Insights when you want additional expert support.
IQVIA empowers organizations to make smarter data driven decisions, optimize data access, and develop crucial new insights. We do this through an integrated offering that includes up-to-date global data, advanced analytic solutions (powered by AI and machine learning), and industry-leading healthcare expertise.
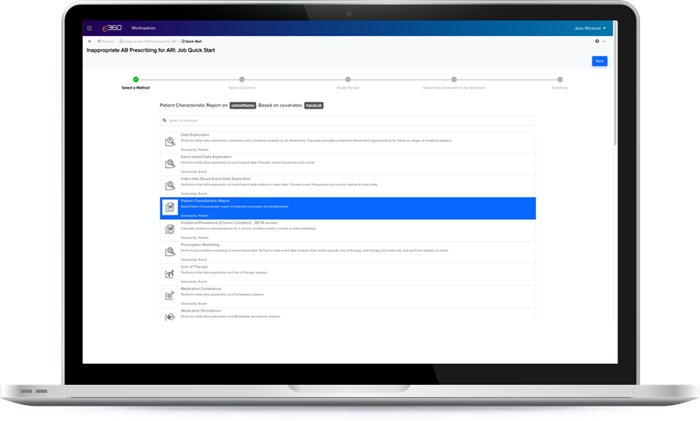
E360® enables faster insight generation from feasibility to completed study with an intuitive user interface, pre-defined queries and guided tools. You don’t have to be a data scientist… no coding required!
Leverage E360® Analytics Workbench to:
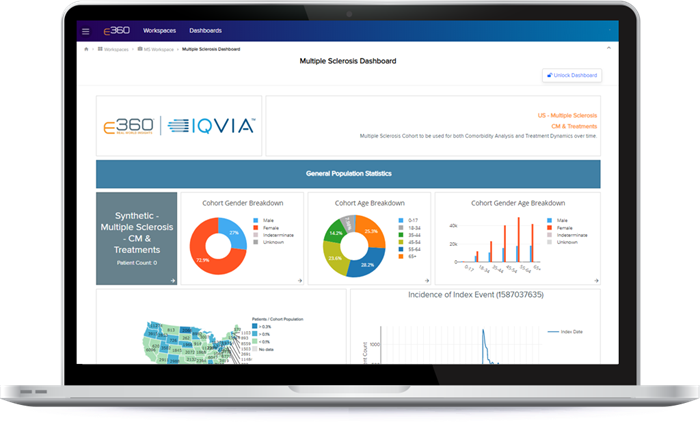
Communicate seamlessly using E360® collaboration capabilities with auto-generated visualizations, reports and dashboards that enable you to create tailored views to highlight key findings, use built-in themes to customize the appearance and include custom images and text.
Enhance stakeholder interactions, allowing you to better:

Real World Data (RWD), patient data that originates from real world settings delivers powerful insights to healthcare stakeholders
Leveraging insights made possible by AI technology, Medical Affairs professionals are fostering better connections with healthcare professionals.
IQVIA’s RWD analytics and visualization platform delivers fast answers to support critical decisions
One stop self-service shop to quickly identify the right Real World Data for your research project
Real world data (RWD) and real world evidence (RWE) are playing an increasing role in health care decisions, with the 21st Century Cures Act, placing additional focus on the use of these types of data to support regulatory decision making.
E360™ Clinical Development is the industry leading SaaS technology platform offering best in class real-world insights to inform protocol design and state-of-the-art analytical tools for effective trial optimization.
Mental Health Consequences of Covid-19: The role of social determinants of health research brief