Introduction
Rare diseases, as the name itself implies, are diseases or conditions that are individually rare and thus have low prevalence rates, with exact definitions varying across different regions and countries. However, collectively, rare diseases are common, affecting approximately 10% of the global population1.
Yet, despite the huge market potential and a wide variety of therapeutic categories for development, drugs intended for the treatment of these diseases, otherwise known as orphan drugs, are scarce and grossly inadequate. In fact, 95% of the 10,000 distinct rare diseases still lack approved treatment options2, highlighting an urgent unmet medical need for orphan drugs. Figure 1 below gives a high-level overview on the definition of rare diseases and orphan drugs targeting those.
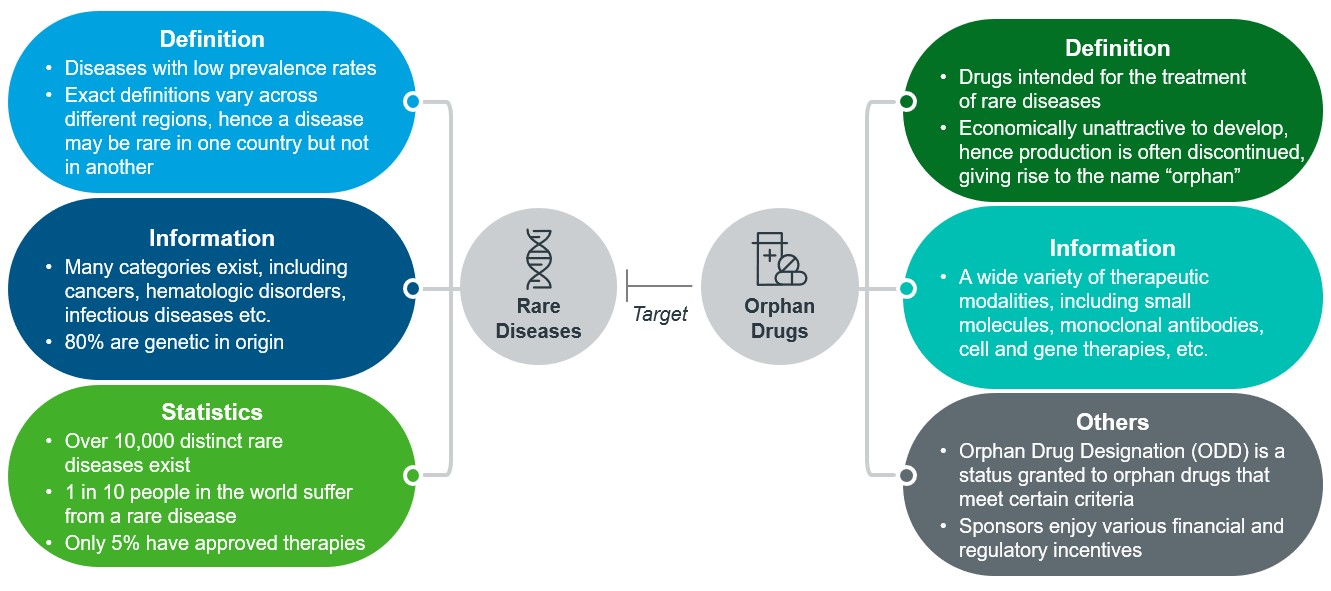
Figure 1: An illustration detailing more information on rare diseases and orphan drugs
Challenges faced in the development of orphan drugs
As with many products, the scarcity of orphan drugs can be explained by a simple cost-benefit economical model. Most, if not all, orphan drugs face significant regulatory challenges3,4 that are unique to them, notably:
- Poor understanding on the natural history and pathophysiology of rare diseases
- Lack of established clinical outcome measures and well-defined endpoints
- Limited and geographically diverse patient populations for study recruitment
- Ethical and feasibility concerns for the implementation of placebo arms
As a result, clinical studies for orphan drugs, especially phase 3 multi-regional trials, are often not optimally designed, span long durations, and missing critical data. Consequently, the development process of orphan drugs tends to be much more time-consuming5 and expensive than that of their non-orphan counterparts, with only approximately 17% of all orphan drugs successfully obtaining regulatory approvals and entering the markets6. Thus, given the perceived small customer base, it is simply not financially viable for companies to invest in orphan drug development.
To address this urgent unmet need for orphan drugs and improve patient access, many health authorities around the world have put in place various policies providing regulatory and economic incentives to encourage and accelerate their development (Figure 2), the most prominent example being the orphan drug designation (ODD).
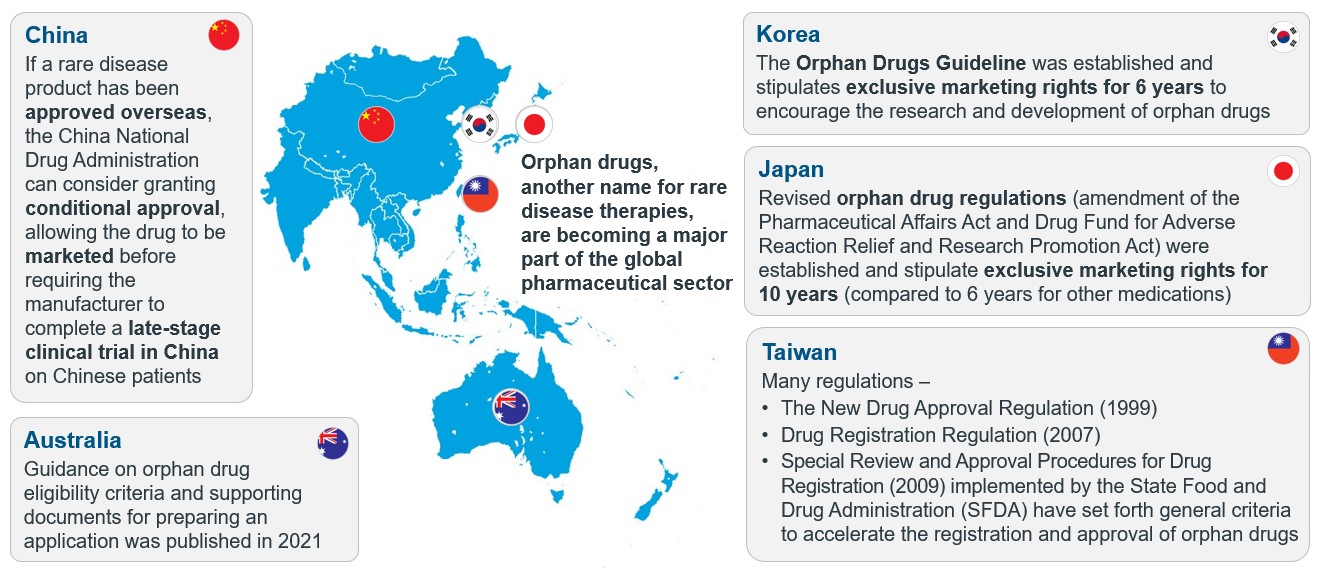
Figure 2: A figure illustrating the evolving regulatory policies and guidelines for orphan drugs
However, while the establishment of such legislatures has certainly improved the situation, the lack of a standardized format has also disharmonized the regulatory framework on rare diseases and orphan drugs across the globe (Figure 3). With different countries each having their own set of policies and guidelines they abide by, this can present a massive source of confusion and significant hurdle for companies in developing and commercializing orphan drugs.
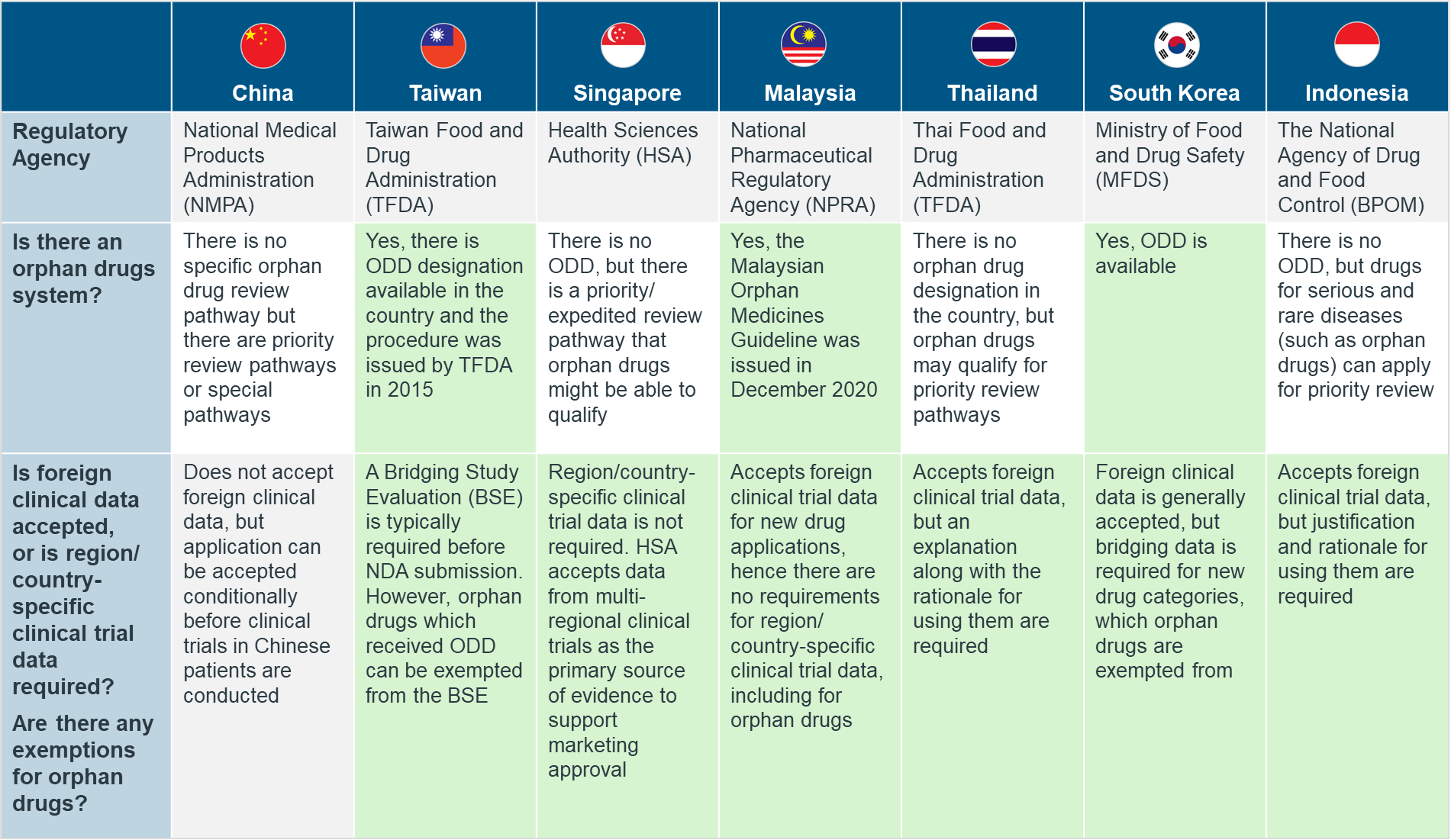
Figure 3: A table detailing the disharmony of orphan drug policies in selected APAC countries
Fortunately, there are strategies companies can take to overcome these obstacles and shorten the development duration and time-to-market for orphan drugs, ensuring early market access and optimal patient access. In this blog, we highlight 2 strategies that are particularly pertinent for pharmaceutical companies considering launching drug products for rare diseases.
Solution 1 – Early regulatory strategy planning
With early regulatory planning7,8, companies can better design clinical trials (e.g., subject profiles, safety requirements for product, etc.) to generate quality data with product registration and approval as the end-goal. by With a regulatory strategy early in place, companies can also facilitate engagement and alignment with relevant stakeholders, anticipate potential questions from health authorities, mitigate risks, as well as streamline the overall drug development process, avoiding unnecessary delays (such as local bridging trials which may be needed due to ethnicity concerns) and ensuring quicker market access. Additionally, starting regulatory planning early is also especially advantageous for manufacturers intending to launch their products across multiple countries in parallel, where requirements for drug approvals may differ from market to market.
In Figure 4, we have included an illustrative case study of the different regulatory challenges faced by a company in registering a rare disease product in various countries, highlighting the importance of early regulatory planning. Our recommendation is to start with a regulatory intelligence to screen and select markets for immediate registration feasibility based on each country’s requirements, health authority discussions, local country epidemiology, prevalence and unmet medical need based on competitive analysis, together with regulatory requirements.
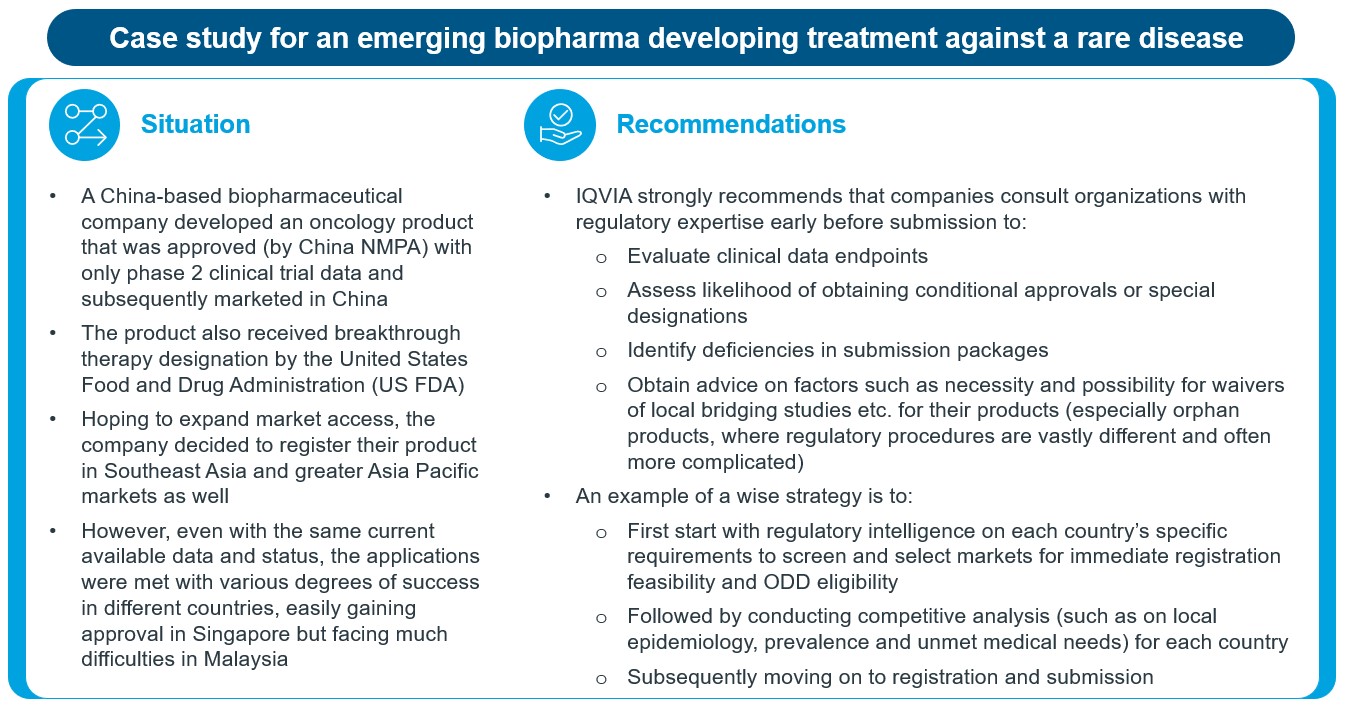
Figure 4: A case study emphasizing the importance of early regulatory planning
Furthermore, gathering regulatory intelligence in advance can also be beneficial for identifying programs or expedited pathways that drug candidates may be eligible for. For instance, ODD may provide sponsors with various perks that could unlock faster approval routes, including priority reviews and less stringent clinical or regulatory requirements (e.g., local clinical trial waivers, early evidence data packages etc.), depending on the country of application. While ODD can be applied any time during the product development, early application yields the maximum benefits, as procedures early in the process may also be shortened or skipped entirely (Figure 5). Therefore, the importance of early regulatory planning in reducing the overall drug development timeline cannot be overlooked.
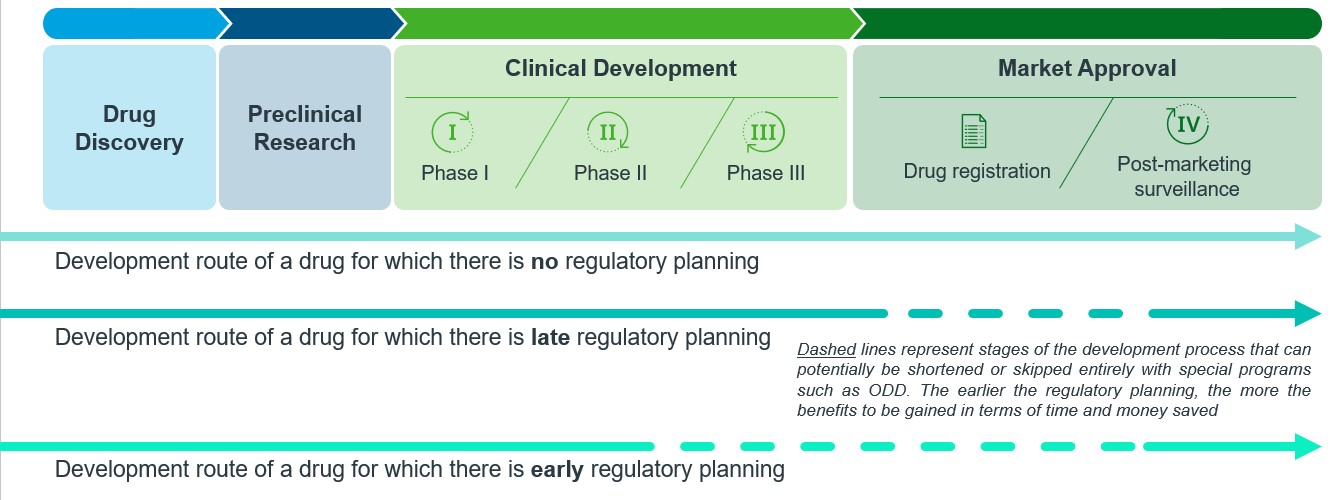
Figure 5: Drug developmental route of drugs without and with early regulatory planning
Solution 2 – Taking an evidence-based approach
Additionally, real-world evidence (RWE) can also be utilized to accelerate orphan drug development, especially at the clinical stage. RWE is the collection of clinical evidence relating to the potential benefits and risks of products, whether they are pharmaceuticals or medical devices9. It is mainly derived from real-world data (RWD), which is generated from a wide range of sources, including, but not limited to, electronic health records, electronic medical records, health surveys, patient registries as well as wearable devices. As such, RWE is an extremely useful means for acquiring more scientific evidence.
In fact, on top of its original role in post-marketing surveillance, RWE is increasingly being utilized in the pre-approval regulatory phase to inform clinical trial design and even serve as an external control group for single arm trials10, which is usually the case with orphan drugs. Furthermore, with more and more policies being continuously put in place to guide its utilization, there is no doubt that health authorities will gradually become more receptive towards using RWE for decision-making in the regulatory space, although with the frameworks being at various stages of development in different countries, the road to international harmonization is still a long one. Hence, taking advantage of RWE, especially when coupled with early planning, could hold the key to early market access for companies launching orphan drugs.
Our recommendations
The rare diseases and orphan drugs regulatory landscape is a volatile one with continuous changes to current regulatory guidelines, which requires companies to constantly rethink their regulatory strategies for orphan drug development and launches. As such, IQVIA strongly recommends companies to start regulatory planning and establish appropriate partnerships as early as possible in order to develop the most time- and cost-efficient plans for registering their products.
Navigate the ever-evolving regulatory landscape with ease and ensure the most optimal approval route for your product launches in various APAC countries and even globally, ultimately accelerating market entry and patient access to life-saving medicines.
For more information on orphan drugs or how we can provide you with regulatory (as well as commercial and reimbursement) support throughout the whole product development life-cycle journey, please contact us.
References
1“Global Data Access for Solving Rare Disease: A Health Economics Value Framework,” World Economic Forum, 26-Feb-2020. [Online]. Available: It's time to share the data on rare disease | World Economic Forum (weforum.org).
2“Rare Disease Facts,” Global Genes. [Online]. Available: RARE Disease Facts - Global Genes.
3S. B. C. Nick, D. D. A. Hughes, and S. G. R. Macaulay, “Rising to the Challenges of Developing Rare Disease Treatments,” DIA Global Forum. [Online]. Available: Rising to the Challenges of Developing Rare Disease Treatments (diaglobal.org).
4A. Khachatryan, S. H. Read, and T. Madison. “External control arms for rare diseases: building a body of supporting evidence,” Journal of Pharmacokinetics and Pharmacodynamics, 24-Apr-2023. [Online]. Available: https://link.springer.com/article/10.1007/s10928-023-09858-8.
5N. Walker, “Where Do Orphan Drugs Go From Here?” Pharma’s Almanac, 24-May-2019. [Online]. Available: https://www.pharmasalmanac.com/articles/where-do-orphan-drugs-go-from-here.
6K. L. Miller, L. J. Fermaglich, and J. Maynard, “Using four decades of FDA orphan drug designations to describe trends in rare disease drug development: substantial growth seen in development of drugs for rare oncologic, neurologic, and pediatric-onset diseases,” Orphanet Journal of Rare Diseases, 9-Jun-2021. [Online]. Available: Using four decades of FDA orphan drug designations to describe trends in rare disease drug development: substantial growth seen in development of drugs for rare oncologic, neurologic, and pediatric-onset diseases | Orphanet Journal of Rare Diseases | Full Text (biomedcentral.com).
7S. Blackburn, J. J. Doyle, J. Hecht, L. Hughes, C. Jackson, J. Perry, and S. Tansey, “Rare Disease: An integrated, patient-centred approach to research and commercialization,” Quintiles. [Online]. Available: e23711b6a82175d7013ce71f1d142a646a9b8b42.pdf (d3kex6ty6anzzh.cloudfront.net).
8L. D’Angelo, “Emerging Strategies for Pre-Launch Access Programs,” Clinical Leader, 1-Nov-2018. [Online]. Available: Emerging Strategies For Pre-Launch Access Programs (clinicalleader.com).
9K. Jayasundara, A. Hollis, M. Krahn, M. Mamdani, J. S. Hoch, and P. Grootendorst, “Estimating the clinical cost of drug development for orphan versus non-orphan drugs,” Orphanet Journal of Rare Diseases, 10-Jan-2019. [Online]. Available: https://ojrd.biomedcentral.com/articles/10.1186/s13023-018-0990-4.
10Parexel, “Four ways to increase rare disease drug development success.” [Online]. Available: Rare Diseases Playbook.pdf (parexel.com).

















