





























- Locations
- Asia Pacific
- Forging a New Path in Innovative Clinical Trial Design (ICTD) for Cell and Gene Therapies
Cell-based and gene therapy products (CGTPs) are innovative products that primarily target orphan indications and diseases with high unmet needs. The lack of a viable treatment alternative for the diseases that CGTPs aim to target calls for accelerated patient access through the use of more flexible clinical designs and methodologies that can optimize trial design and drug development.
This article will focus on the current landscape of clinical development for CGTPs, with respect to innovative clinical trial designs (ICTDs).
Current pain points of conventional clinical trials for CGTPs
Clinical trials are typically run in three steps:
- The trial is designed.
- The trial is conducted in accordance with the design.
- Data is analyzed post-collection according to a pre-specified analysis plan3.
By allocating enough individuals to experimental and control arms, investigators can study the effect of a given intervention4. Applying the same design to CGTP trials may however be less efficient, due to the reasons as illustrated in Figure 1.
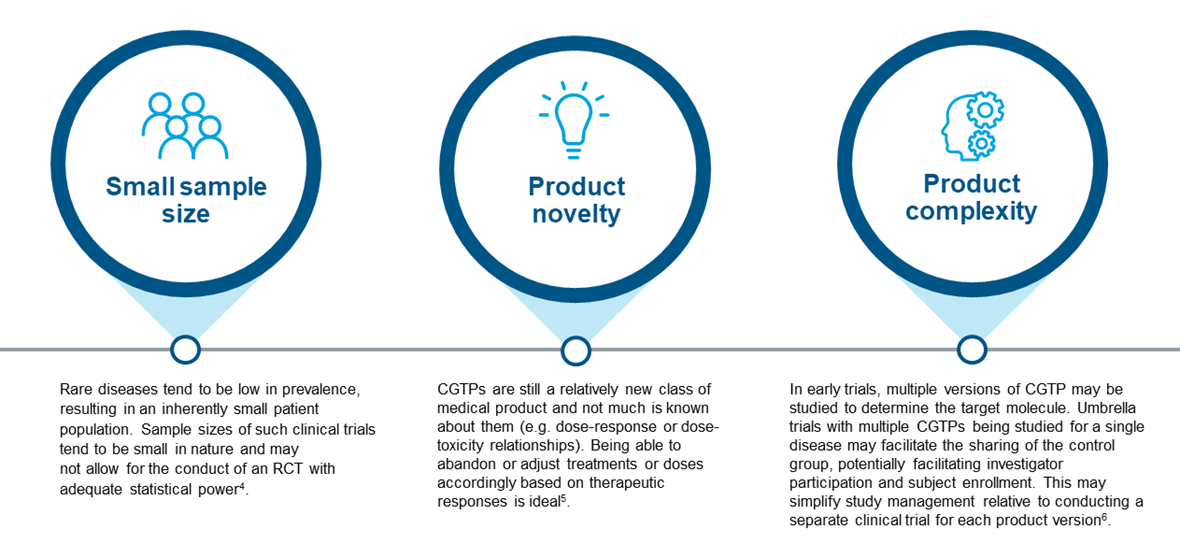
Figure 1. Overarching pain points in traditional clinical trials for CGTPs
Innovative clinical trial designs and the limitations of conventional trials they seek to address
Innovative clinical trial designs (ICTDs), according to the United States Food and Drug Administration (FDA) and National Institute of Health (NIH) respectively, are defined as trials that “have rarely or never been used to date to provide substantial evidence of effectiveness in new drug or biologics license applications”7, and that “represent a deviation from the traditional clinical design”8. It is important to note that the definition of ICTDs can change with time and aspects of essential trial designs will vary by therapeutic area. ICTDs may come in four types, as depicted in the figure below:
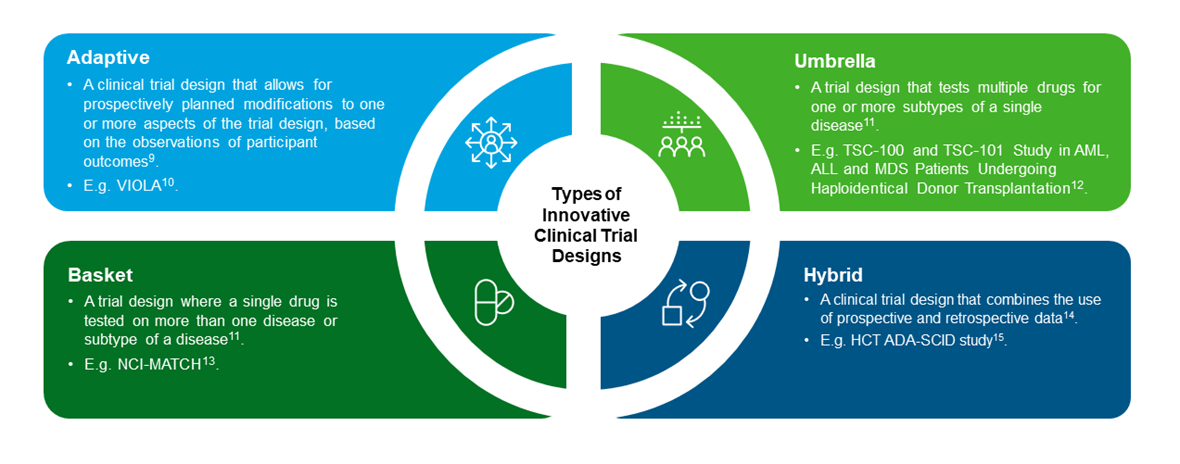
Figure 2. Overview and examples of the different types of clinical trial designs
The type of trial design to adopt is dependent on factors such as the phase of drug development that they are used, the outcome as well as recruitment effects. Umbrella trials for instance, investigate the safety and efficacy of several Investigational Medical Products (IMPs) in a single population, and can be used at any stage of development, while basket trials investigate the safety and efficacy of an IMP or a combination of IMPs across a variety of populations, and are typically used early in drug development.
ICTDs are able to address the drawbacks of conventional clinical trial designs through the following ways, as detailed in Figure 3:
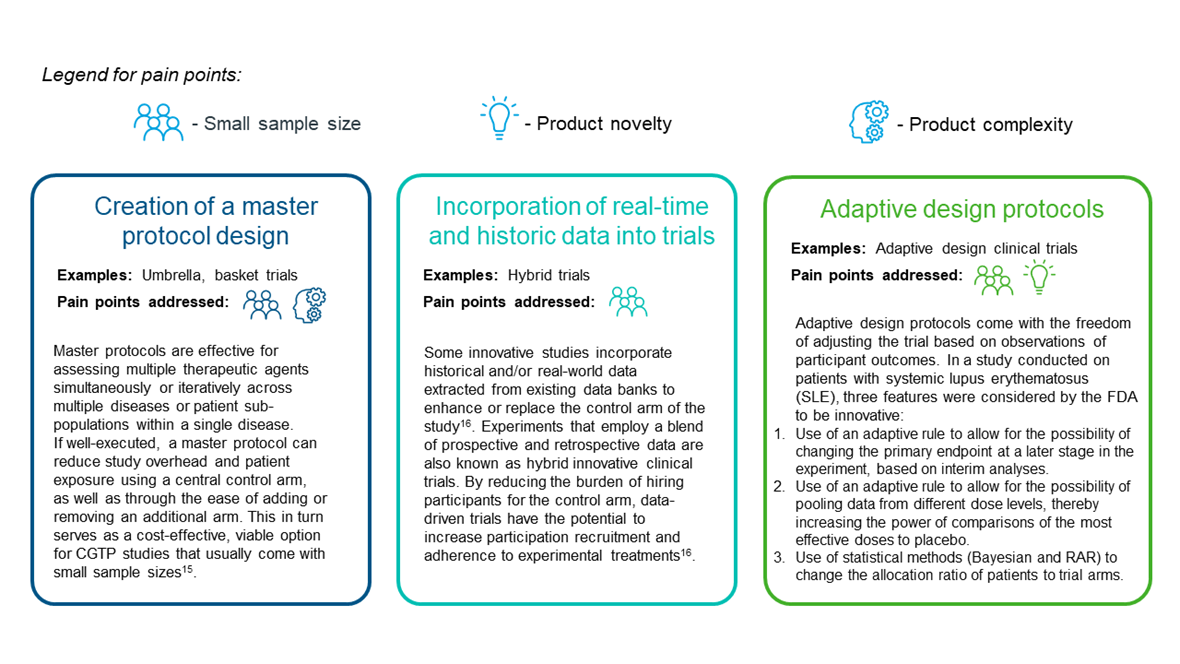
Figure 3. Selected features of ICTDs and how they address the pain points of clinical trials
Adaptive clinical trials in particular, are of increasing interest as changes to treatment arms often allocate subjects to superior treatment regimens. This in turn minimizes the number of participants exposed to ineffective or toxic doses, thereby increasing the likelihood of achieving the study objective in eventuality18.
The way forward for innovative clinical trial designs: Three things to pay attention to
1. The growing demand to digitize clinical trials
Companies face growing pressure to reduce development costs and increase participation in clinical trials. Digital technology can be used as a tool to improve participant recruitment and retention, as well as to facilitate data collection and analysis in the ways as illustrated below19:
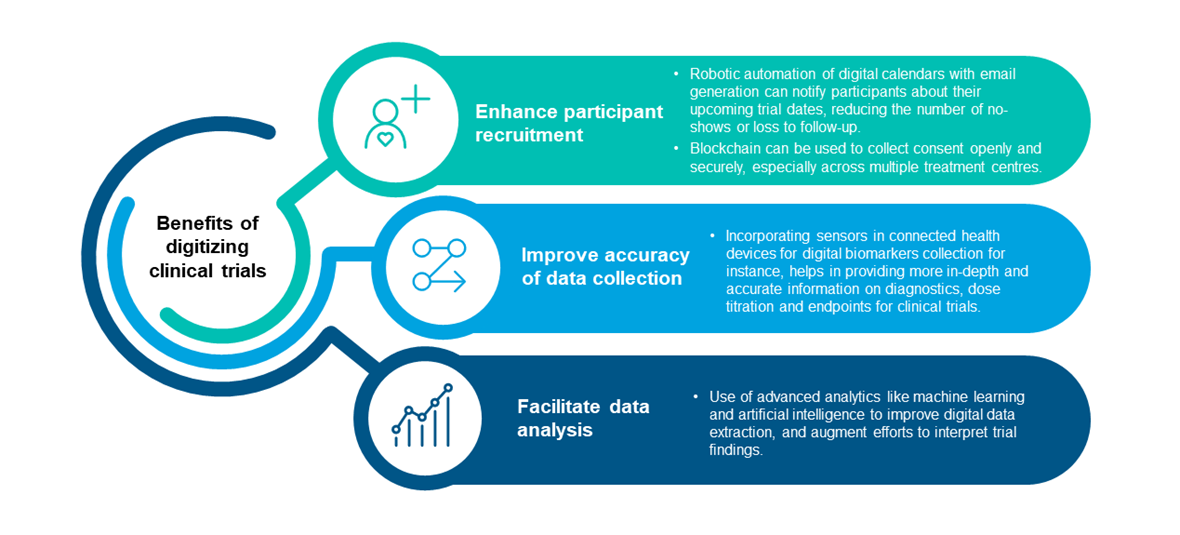
Figure 4. Benefits of digitizing clinical trials
Incorporating digital technologies into ICTDs will be especially helpful in optimizing recruitment targets for rare disease trials with smaller patient population. Furthermore, digital technologies in the form of devices and advanced analytics software can help improve the accuracy of data collection and facilitate data analysis, thereby assisting in the generation of robust clinical data in the most cost- and time-effective way possible. Coupling both digital and innovative approaches may therefore help in resolving some of the pain points that come with small-sized CGTP trials.
2. Differences in the level of guidance between countries
As innovative clinical trial designs are increasingly used, coming up with guidance to provide Health Authorities’ stances are important. To date, the FDA and European Medicines Agency (EMA) have released work plan guidance (FDA: Interacting with the FDA on Complex Innovative Trial Designs for Drugs and Biological Products: Guidance for Industry and Innovation in Clinical Trial design | EMA: ACT EU multi-annual workplan 2022-2026) on the subject matter.
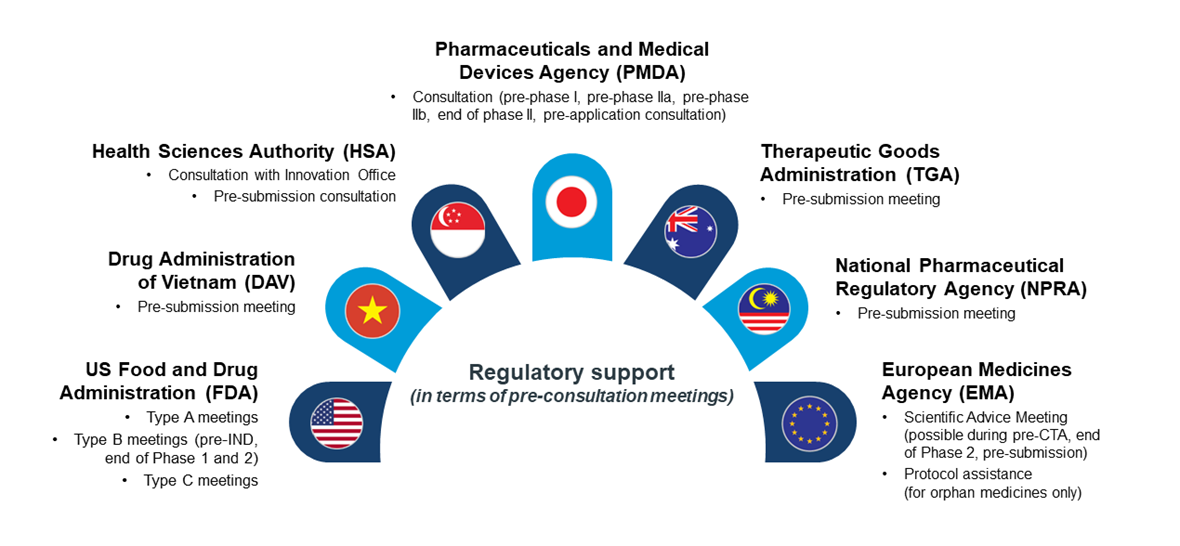
Figure 5. Regulatory support (in terms of pre-consultation meetings) provided by regulatory agencies in selected countries.
The lack of reference could create difficulties for sponsors seeking to conduct innovative clinical trials for eventual approval. Having a one-to-one consultation session to seek alignment with authorities on their expectations when filing for regulatory approval is therefore imperative.
3. Relative novelty and risk of ICTDs
As of to date, little is known about the use of ICTDs for cell and gene therapy products – in the case of adaptive trials, there is a lack of clarity on when they are applicable, what they can or cannot accomplish, what their practical implications are and how their results should be interpreted or reported5. Studies leveraging real world data to provide information on a wider patient population may contain inherent biases in their data sources that could limit their value for establishing causality between drug exposure and outcomes20.
Despite the potential benefits that ICTDs may bring for CGTPs, developers must still exercise due diligence in reviewing the suitability of this mode of trial design for their IMPs. To optimize their chances of succeeding in market access, industry stakeholders are recommended to strive to understand and apply the recommendations of regulatory agencies, as well as to partner with providers who have clinical trial experience for a better understanding of the ICTD landscape.
CGTPS offer hope for the treatment of serious diseases with unmet medical need – expertise is required to navigate the regulatory space to ensure regulatory compliance and access into each market. To find out more about cell-based and gene therapy products across the JAPAC region, please click here.
References:
- Iglesias-Lopez, C., Agustí, A., Vallano, A., & Obach, M. (2021). Current landscape of clinical development and approval of Advanced Therapies. Molecular Therapy - Methods & Clinical Development, 23, 606–618. https://doi.org/10.1016/j.omtm.2021.11.003
- Galli, M.C., and Serabian, M. (2015). Regulatory Aspects of Cell and Gene Therapy Products. Advances in Experimental Medicine and Biology. Volume 871. https://www.royanatmp.com/pdf/Regulatory-Aspects-of-Gene-Therapy-and-Cell-Therapy-Products.pdf
- Friedman FL, Furberg CD, DeMets DL. Fundamentals of clinical trials, 4th ed. New York: Springer; 2010.
- Evans, C. H., Ildstad, S. T., & Institute of Medicine (U.S.) (Eds.). (2001). Small clinical trials: Issues and challenges. National Academy Press.
- Pallmann, P., Bedding, A. W., Choodari-Oskooei, B., Dimairo, M., Flight, L., Hampson, L. V., Holmes, J., Mander, A. P., Odondi, L., Sydes, M. R., Villar, S. S., Wason, J. M. S., Weir, C. J., Wheeler, G. M., Yap, C., & Jaki, T. (2018). Adaptive designs in clinical trials: Why use them, and how to run and report them. BMC Medicine, 16(1), 29. https://doi.org/10.1186/s12916-018-1017-7
- Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER). (2021). Studying Multiple Versions of a Cellular or Gene Therapy Product in an Early-Phase Clinical Trial: Draft Guidance for Industry. Studying Multiple Versions of a Cellular or Gene Therapy Product in an Early-Phase Clinical Trial; Draft Guidance for Industry (fda.gov)
- Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER). (2020). Interacting with the FDA on Complex Innovative Trial Designs for Drugs and Biological Products: Guidance for Industry. https://www.fda.gov/media/130897/download
- An introduction and overview of innovative trial design | nhlbi, nih. (n.d.). https://www.nhlbi.nih.gov/events/2018/introduction-and-overview-innovative-trial-design
- Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER). (2019). Adaptive Designs for Clinical Trials of Drugs and Biologics: Guidance for Industry. https://www.fda.gov/media/78495/download
- Mahajan, R., & Gupta, K. (2010). Adaptive design clinical trials: Methodology, challenges and prospect. Indian Journal of Pharmacology, 42(4), 201–207. https://doi.org/10.4103/0253-7613.68417
- Yap C, Billingham LJ, Cheung YK, Craddock C, O’Quigley J. Dose transition pathways: the missing link between complex dose-finding designs and simple decision making. Clin Cancer Res. 2017;23:7440–7.
- Naas, L.A. (2019). Clinical Trial Design: Basket and Umbrella Studies. Lilly Trials. https://trials.lilly.com/en-US/blog/clinical-trial-design-basket-umbrella-studies
- FOCUS4: A flagship trial in colorectal cancer—Department of Oncology. (n.d.). https://www.oncology.ox.ac.uk/blog/focus4-a-flagship-trial-in-colorectal-cancer
- ISPOR Glasgow. (2017). Innovative Clinical Trial Designs: Welcomed by Regulators but What About the Payers? https://www.ispor.org/docs/default-source/presentations/1330.pdf?sfvrsn=56ed4398_1
- Hassan, A., Booth, C., Brightwell, A., Allwood, Z., Veys, P., Rao, K., Hönig, M., Friedrich, W., Gennery, A., Slatter, M., Bredius, R., Finocchi, A., Cancrini, C., Aiuti, A., Porta, F., Lanfranchi, A., Ridella, M., Steward, C., Filipovich, A., … Gaspar, H. B. (2012). Outcome of hematopoietic stem cell transplantation for adenosine deaminase–deficient severe combined immunodeficiency. Blood, 120(17), 3615–3624. https://doi.org/10.1182/blood-2011-12-396879
- Lu, C. (Cindy), Li, X. (Nicole), Broglio, K., Bycott, P., Jiang, Q., Li, X., McGlothlin, A., Tian, H., & Ye, J. (2021). Practical considerations and recommendations for master protocol framework: Basket, umbrella and platform trials. Therapeutic Innovation & Regulatory Science, 55(6), 1145–1154. https://doi.org/10.1007/s43441-021-00315-7
- Devine, C. (2020). Operationalizing complex innovative trial design(Fda’s new approach). Medpace. https://www.medpace.com/operationalizing-complex-innovative-trial-design-fdas-new-approach/
- CID Case Study: A Study in Patients with Systemic Lupus Erythematosus. (n.d.). https://www.fda.gov/media/155404/download
- Clinical Trials: How Technology is Driving Digitisation (n.d.). Cambridge Consultants. https://www.cambridgeconsultants.com/sites/default/files/uploaded-pdfs/Clinical%20trials%20-%20how%20technology%20is%20driving%20digitisation_1.pdf
- Ghadessi, M., Tang, R., Zhou, J., Liu, R., Wang, C., Toyoizumi, K., Mei, C., Zhang, L., Deng, C. Q., & Beckman, R. A. (2020). A roadmap to using historical controls in clinical trials – by drug information association adaptive design scientific working group(Dia-adswg). Orphanet Journal of Rare Diseases, 15(1), 69. https://doi.org/10.1186/s13023-020-1332-x


