





























- Locations
- Asia Pacific
- Navigating the Diverse Cell, Tissue, and Gene Therapy Landscape in Japan and Asia Pacific (JAPAC)
There has been an increasingly wide interest in the use of cell-based and gene therapy products (CGTPs) for the treatment of diseases and physiological conditions due to their potential to address serious, unmet medical needs. CGTPs encompass a wide range of products – from minimally manipulated skin grafts and blood transfusions to Advanced Therapy Medicinal Products such as cell therapy, gene therapy, tissue engineering products etc. [1]. As most regulatory agencies adopt a risk-based approach, pre-approval is not required for minimally manipulated / homologous products. This article will then focus on the regulations for the approval of non-minimally manipulated and non-homologous use CGTPs.
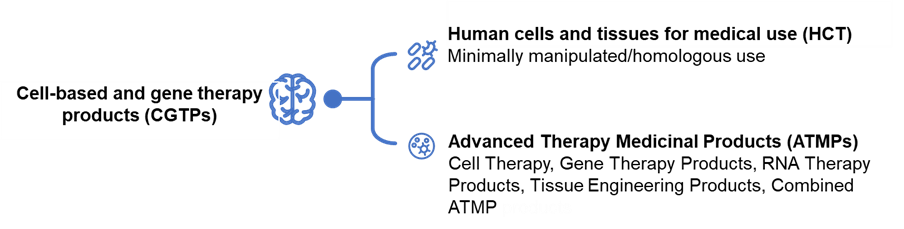
Figure 1. Types of cell and gene therapies
Need for fit-for-purpose regulations
CGTPs are unique from traditional pharmaceutical products as they involve the introduction of live materials into a patient’s body, which comes with certain risks. For instance, patients who are treated with allogeneic cell therapy or in-vivo gene delivery method encounter a higher risk of immunogenicity [2]. To ensure patients’ safety, regulatory requirements of a minimum safety standard for CGTPs have been imposed. However, due to the high batch-to-batch variability in starting material of autologous cell therapies, there is no one-size-fits-all solution. Greater flexibility will need to be accorded in the regulations and for products to be potentially reviewed on a case-by-case basis [3].
The past five years have seen an exponential increase in the number of approvals for CGTPs, in particular for genetically modified cell therapies such as autologous CAR T-cell therapies. Hence, coming up with fit-for-purpose regulations that can address the unique sensitivities of cell and gene therapy products is of increasing importance. The figure below illustrates the increase in regulatory approvals for the different modes of cell, gene, and RNA therapies across a span of seven years:
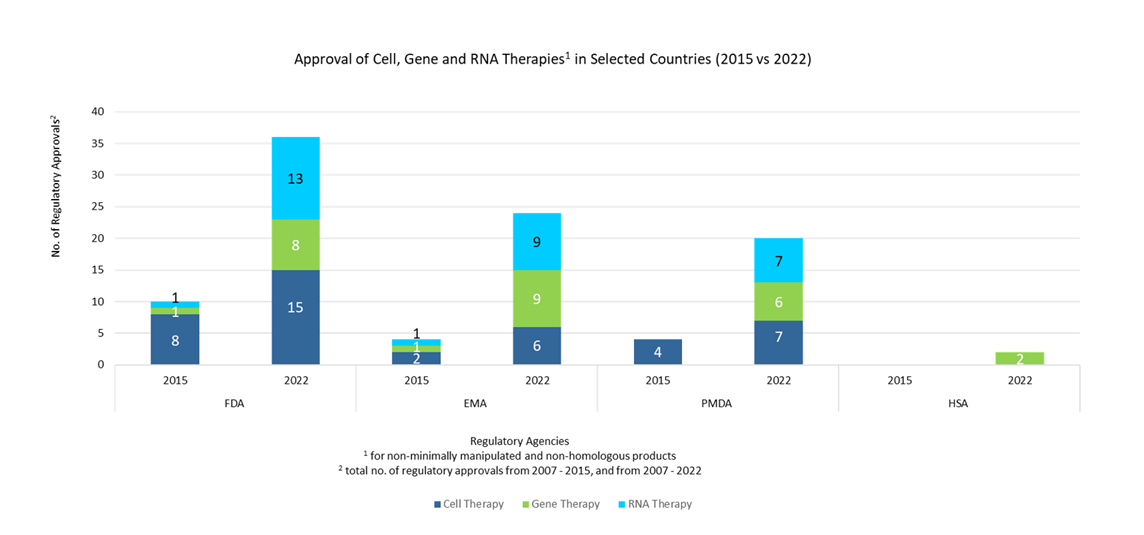
Figure 2. Approval of number of cell, gene, and RNA therapies in selected countries / region over 7 years [4, 5, 6, 7]
Differences in regulatory frameworks between selected JAPAC countries
Due to the relative novelty of CGTPs, cell and gene therapy frameworks are still in their nascent stages of development in some countries. The lag in creating a viable regulatory framework has resulted in a divergence in frameworks between countries like the United States (US), Europe (EU) and Singapore that have well-established CGTP frameworks, versus emerging economies like Vietnam and Indonesia that lack one.
Harmonisation of CGTP frameworks between countries with an existing framework is also a challenge as CGTPs are classified differently and are thus subjected to different controls and approval pathways in different countries. Regulatory classification and control-wise, the European Medicines Agency (EMA) for instance, regulates ATMPs instead of CGTPs, and classifies ATMPs into four major groups: gene therapy, somatic cell therapy, tissue-engineered therapy and combined advanced therapy. HSA on the other hand, classifies CGTPs into two risk classes based on the extent of manipulation. All in all, the differences in cell and gene therapy regulatory frameworks across multiple countries can be challenging for pharmaceutical companies who are seeking market access in more than one country in parallel.
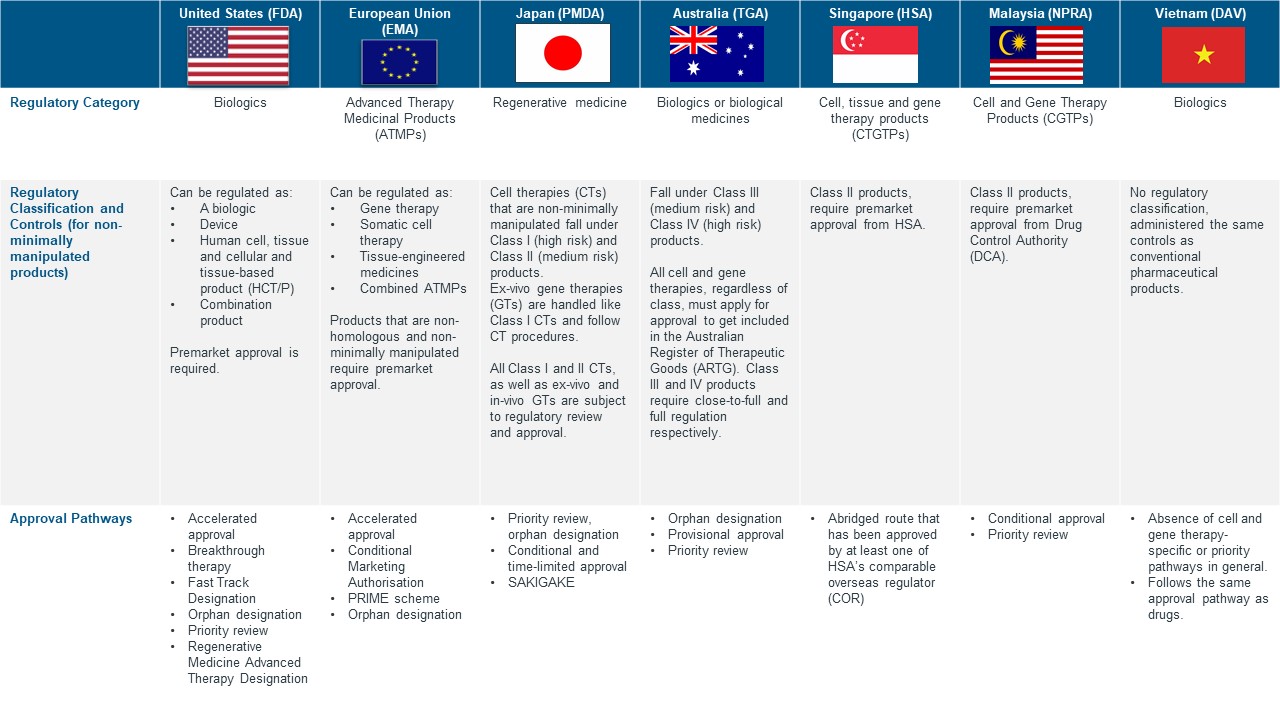
Figure 3. CGTP Regulatory Framework in US, EU and selected JAPAC Countries [3, 8]
*US and EU are typically used as reference countries in selected APAC countries
The future of CGTPs for the JAPAC region
With the rapid development of CGTPs, establishing harmonised regulatory frameworks that can address their unique characteristics is important. The existence of cross-country industry/regulators coalitions (e.g. APEC RHSC Advanced Therapies Industry Coalition Coordinators, Standards Coordinating Body and Alliance for Regenerative Medicine) can provide a platform for countries in the Asia-Pacific region to share CGTP-related information and good manufacturing practices with one another, as well as to facilitate the establishment of Mutual Recognition Agreements (MRAs). This bodes well for the harmonisation of CGTP regulations in the JAPAC region.
CGTPs offer hope for the treatment of serious diseases with unmet medical need - expertise is required to navigate the regulatory space to ensure regulatory compliance and access into each market. To find out more about cell-based and gene therapy products across the JAPAC region, please click here.
References:
[1]. World Health Organisation (2021). WHO considerations on Regulatory Convergence of Cell and Gene Therapy Products. who-public-consultation_cgtp-white-paper_16_dec_2021.pdf
[2]. US Food and Drug Administration (2020). Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use Guidance for Industry and Food and Drug Administration Staff. www.fda.gov/media/109176/download
[3]. Galli, M.C., and Serabian, M. (2015). Regulatory Aspects of Cell and Gene Therapy Products. Advances in Experimental Medicine and Biology. Volume 871. Regulatory-Aspects-of-Gene-Therapy-and-Cell-Therapy-Products.pdf (royanatmp.com)[4]. Research, Center for Biologics Evaluation. Approved Cellular and Gene Therapy Products. FDA, June 2022. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.
[5]. Iglesias-Lopez, Carolina, et al. Current Landscape of Clinical Development and Approval of Advanced Therapies. Molecular Therapy – Methods & Clinical Development, vol. 23, Dec. 2021, pp.606-18. https://doi.org/10.1016/j.omtm.2021.11.003.
[6]. Sakurai, A. (n.d.). Regenerative Medicine and Gene Therapy in Japan. PMDA. 000241234.pdf (pmda.go.jp)
[7]. HSA | Class 2 CTGTP Registration. https://www.hsa.gov.sg/ctgtp/registration
[8]. NPRA. Guidance Document and Guidelines for Registration of Cell and Gene Therapy Products (CGTPs) in Malaysia. https://www.npra.gov.my/index.php/en/guideline-bio/1527314-guidance-document-and-guidelines-for-registration-of-cell-and-gene-therapy-products-cgtps-in-malaysia-2.html


