-
Americas
-
Asia & Oceania
-
A-I
J-Z

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more -
Middle East & Africa

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more
Regions
-
Americas
-
Asia & Oceania
-
Europe
-
Middle East & Africa
-
Americas
-
Asia & Oceania
-
Europe
Europe
- Adriatic
- Belgium
- Bulgaria
- Czech Republic
- Deutschland
- España
- France
- Greece
- Hungary
- Ireland
- Israel
- Italia

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more -
Middle East & Africa

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more
SOLUTIONS
-
Research & Development
-
Real World Evidence
-
Commercialization
-
Safety & Regulatory Compliance
-
Technologies
LIFE SCIENCE SEGMENTS
HEALTHCARE SEGMENTS
- Information Partner Services
- Financial Institutions
- Public Health and Government
- Patient Associations
- Payers
- Providers
THERAPEUTIC AREAS
- Cardiovascular
- Cell and Gene Therapy
- Central Nervous System
- GI & Hepatology
- Infectious Diseases and Vaccines
- Oncology
- Pediatrics
- Rare Diseases
- View All

Impacting People's Lives
"We strive to help improve outcomes and create a healthier, more sustainable world for people everywhere.
LEARN MORE
Harness the power to transform clinical development
Reimagine clinical development by intelligently connecting data, technology, and analytics to optimize your trials. The result? Faster decision making and reduced risk so you can deliver life-changing therapies faster.
Research & Development OverviewResearch & Development Quick Links

Real World Evidence. Real Confidence. Real Results.
Generate and disseminate evidence that answers crucial clinical, regulatory and commercial questions, enabling you to drive smarter decisions and meet your stakeholder needs with confidence.
REAL WORLD EVIDENCE OVERVIEWReal World Evidence Quick Links

See markets more clearly. Opportunities more often.
Elevate commercial models with precision and speed using AI-driven analytics and technology that illuminate hidden insights in data.
COMMERCIALIZATION OVERVIEWCommercialization Quick Links

Service driven. Tech-enabled. Integrated compliance.
Orchestrate your success across the complete compliance lifecycle with best-in-class services and solutions for safety, regulatory, quality and medical information.
COMPLIANCE OVERVIEWSafety & Regulatory Compliance Quick Links

Intelligence that transforms life sciences end-to-end.
When your destination is a healthier world, making intelligent connections between data, technology, and services is your roadmap.
TECHNOLOGIES OVERVIEWTechnology Quick Links
CLINICAL PRODUCTS
COMMERCIAL PRODUCTS
COMPLIANCE, SAFETY, REG PRODUCTS
BLOGS, WHITE PAPERS & CASE STUDIES
Explore our library of insights, thought leadership, and the latest topics & trends in healthcare.
DISCOVER INSIGHTSTHE IQVIA INSTITUTE
An in-depth exploration of the global healthcare ecosystem with timely research, insightful analysis, and scientific expertise.
SEE LATEST REPORTSFEATURED INNOVATIONS
-
IQVIA Connected Intelligence™
-
IQVIA Healthcare-grade AI™
-
Human Data Science Cloud
-
IQVIA Innovation Hub
-
Decentralized Trials
-
Patient Experience powered by Apple
WHO WE ARE
- Our Story
- Our Impact
- Commitment to Public Health
- Code of Conduct
- Environmental Social Governance
- Privacy
- Executive Team
NEWS & RESOURCES

Unlock your potential to drive healthcare forward
By making intelligent connections between your needs, our capabilities, and the healthcare ecosystem, we can help you be more agile, accelerate results, and improve patient outcomes.
LEARN MORE
IQVIA AI is Healthcare-grade AI
Building on a rich history of developing AI for healthcare, IQVIA AI connects the right data, technology, and expertise to address the unique needs of healthcare. It's what we call Healthcare-grade AI.
LEARN MORE
Your healthcare data deserves more than just a cloud.
The IQVIA Human Data Science Cloud is our unique capability designed to enable healthcare-grade analytics, tools, and data management solutions to deliver fit-for-purpose global data at scale.
LEARN MORE
Innovations make an impact when bold ideas meet powerful partnerships
The IQVIA Innovation Hub connects start-ups with the extensive IQVIA network of assets, resources, clients, and partners. Together, we can help lead the future of healthcare with the extensive IQVIA network of assets, resources, clients, and partners.
LEARN MORE
Proven, faster DCT solutions
IQVIA Decentralized Trials deliver purpose-built clinical services and technologies that engage the right patients wherever they are. Our hybrid and fully virtual solutions have been used more than any others.
LEARN MORE
IQVIA Patient Experience Solutions powered by Apple
Empowering patients to personalize their healthcare and connecting them to caregivers has the potential to change the care delivery paradigm. IQVIA and Apple are collaborating to bring this exciting future of personalized care directly to devices patients already have and use.
LEARN MOREWORKING AT IQVIA
Our mission is to accelerate innovation for a healthier world. Together, we can solve customer challenges and improve patient lives.
LEARN MORELIFE AT IQVIA
Careers, culture and everything in between. Find out what’s going on right here, right now.
LEARN MORE
WE’RE HIRING
"Improving human health requires brave thinkers who are willing to explore new ideas and build on successes. Unleash your potential with us.
SEARCH JOBS- Insights
- The IQVIA Institute
- Reports and Publications
- Reports
- Lifetime Trends in Biopharmaceutical Innovation
About the Report
Over the past 20 years, over 700 new active substances (NASs) have successfully been discovered, developed and authorized by regulatory bodies for use with patients globally. This report profiles the NASs launched in the U.S. over the past 20 years and measures the length of a molecule’s lifetime from patent filing to launch and eventual patent expiry. It also explores the significant variations in this lifetime when viewed by molecule characteristics such as therapy area, orphan drug status, and the type of companies involved in the development and marketing. These issues and trends highlight important implications for investors and manufacturers.
Summary
A total of 667 innovative biopharmaceuticals launched in the U.S. over the past 20 years, 1996-2015, bringing new treatment options to patients. After a low point of only 19 NAS launches in 2008, the number has steadily increased and reached 47 in 2015. The characteristics of biopharmaceutical innovation have also evolved over the past two decades, with increases in the share of cancer treatment launches from 11% to 28%, orphan indications from 21% to 42% and biologic launches.
The time between when a medicine is discovered and when the originator ceases to receive significant revenues can be considered its lifetime. The average time from initial patent filing until launch for all molecules studied is 12.8 years, while the time from a product’s launch until the expiration of its patent or other form of exclusivity is just over 13.5 years and has been steadily declining. The combination of these factors are contributing to a decline in economic returns from investment in research and development, although these remain positive.
The majority of drug launches achieve modest levels of average annual sales in their first five years on the market, although a relatively small number of outlier products garner significantly higher sales. A growing percentage of products are taking longer than five years to reach their peak sales, which may reflect slower progress to reach the patients who will benefit from the new medicines.
Key Findings
The Highest Proportion of NASs Launched 1996–2015 Target Oncology and Communicable Diseases
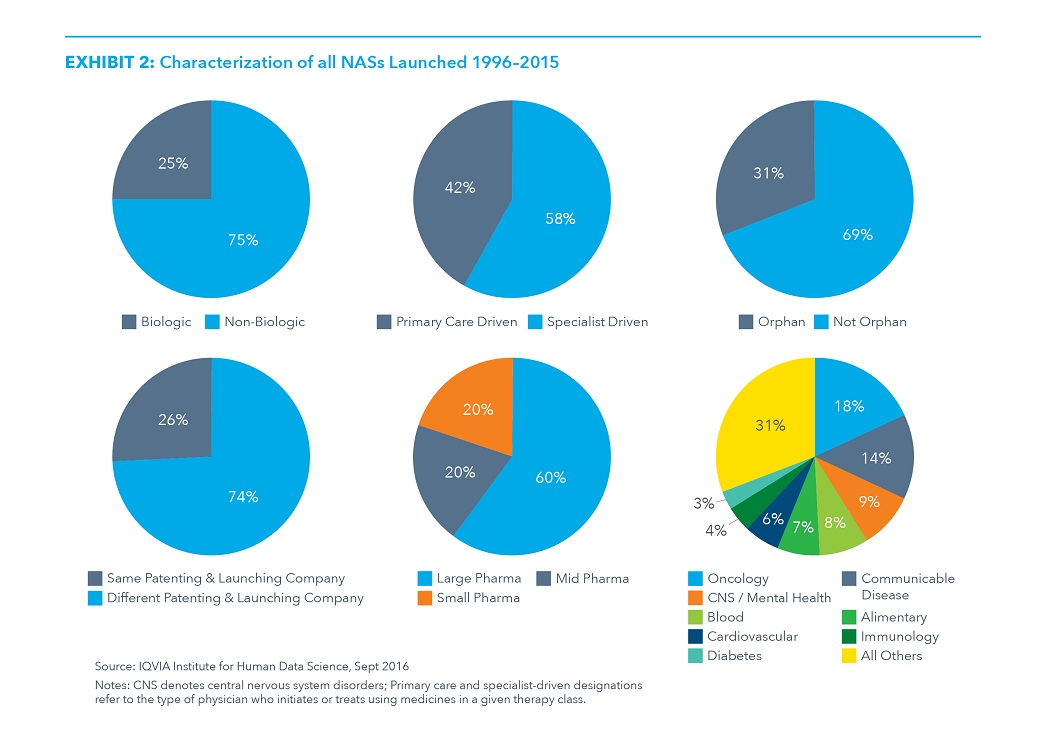
- Oncology drugs accounted for 18% of NASs, as the number of tumor types with highly effective treatments has increased dramatically over 20 years.
- NASs for CNS disorders and mental health contributed 9% to the launches during the analyzed period, while cardiovascular and anti-diabetes drugs make up 6% and 3% of launches, respectively.
- Of the NASs, almost 60% are primarily prescribed to patients by specialists rather than primary care physicians, about a quarter are biologics, and almost one third have one or more orphan designations granted by the FDA.
- Nearly three quarters of new medicines were patented by a different entity than the company that registered the drug with the FDA for marketing in the U.S.
The Average Time from Patent Filing until Launch in the U.S. Market for NASs Launched 1996 to 2015 is 12.8 Years
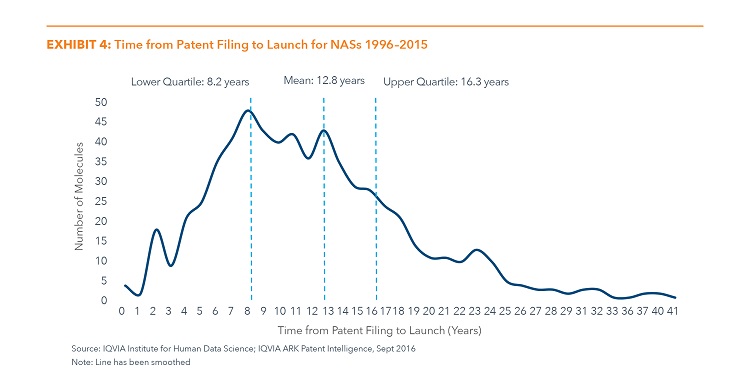
- Some NASs have short patent to launch times with patents filed close to their launch date. This can occur, for example, when the molecule is a naturally occurring substance or when the original patent was filed in another country.
- In other cases, many years or even decades elapse between a drug’s initial patent and its eventual launch. This can reflect, for example, molecules which were patented and whose development was left dormant for a period.
- Over the past 20 years, the average time from patent filing to launch has also varied for each year’s cohort of new drugs from a low of 10 years 8 months in 1998 to a high of 17 years 7 months for the 2008 cohort.
The Average Time from Launch until Patent Expiry in the U.S. Market for NASs Launched 1996 to 2015 is 13.5 Years
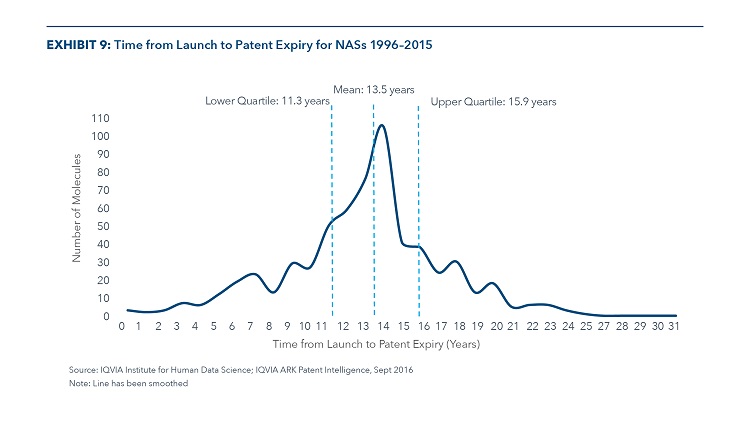
- Patents in the U.S. are granted to protect intellectual property for a period of 20 years from the time of filing, but other forms of exclusivity can be granted. Subsequent patent filings related to a molecule or delivery device also may have a term of twenty years from the date of filing and extend the protected time relative to the original patent.
- Due to delays in clinical trials or setbacks in regulatory reviews, some products reach the market with substantially less of their patent lives remaining than others.
- The majority of NAS reach the market with more than 10 years remaining under patent protection.
Only a Small Share of NASs See High Average Annual Sales in the First Five Years
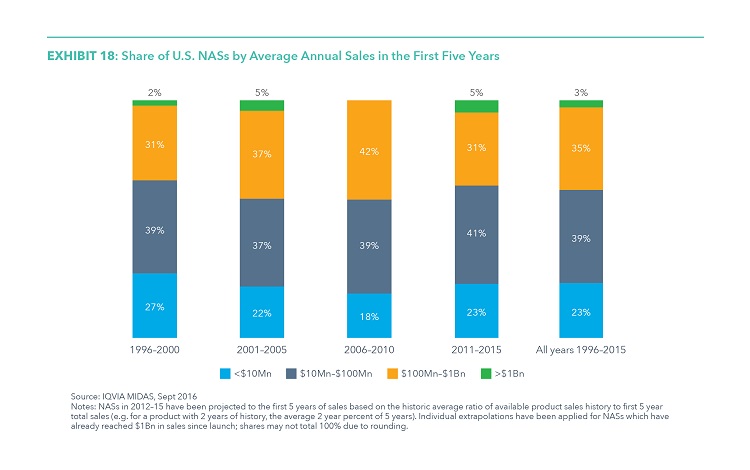
- The majority of drug launches achieve very modest levels of average annual sales in their first five years on the market.
- From 1996–2015, 62% of the launches averaged less than $100 million in average annual sales during their initial five years following launch, suggesting that substantial lifetime returns are exceedingly rare.
- Only 19 drugs have reached the $1 billion in annual sales mark within their first five years on the market over the past 20 years, but 9 of these were launched in the past five years, including the only four drugs that exceeded $3 billion in annual sales within five years of their launch, and among these two notable hepatitis C drugs.











