Access new resources for advanced therapy development, from candidate identification through market authorization.






















- Blogs
- Gearing for Success in Cell and Gene Therapy
Part 1: Introduction
"We used to think that our fate was in our stars, but now we know that … our fate is in our genes"
– James Watson
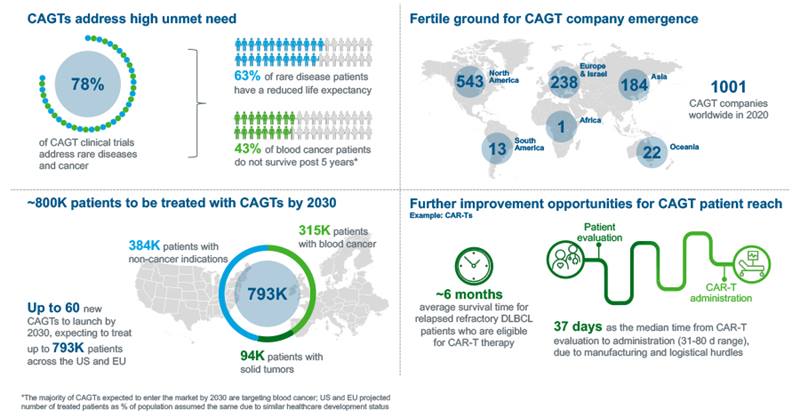
Great strides have been made by many players in Cell and Gene Therapy (CAGT) over the last few decades. We have moved from hype and uncertainty to reality, with patients getting access to life-saving therapies for a multitude of different diseases from rare, inherited neurological diseases to the most advanced stages of cancer. While this journey has seen a growing number of success stories with more than 1000 CAGT-focused companies in 2020, the road has been, and continues to be arduous. For both manufacturers and patients’, the journey continues to resemble the Silk Road of the Middle Ages, a long and arduous journey marred with many dangers leading to a high chance of failure, but ultimately highly rewarding for those who can navigate the risks to completion.
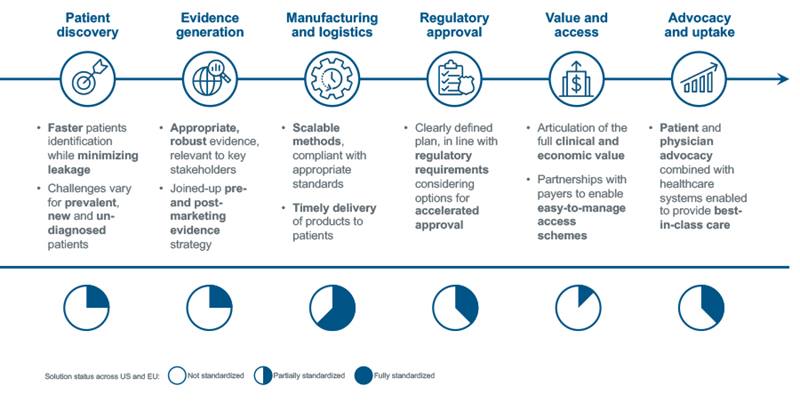
IQVIA evaluated the approaches taken from discovery to commercialization of over 30 companies developing CAGT medicines to distill the six building blocks for CAGT success. CAGT developers must work with all of these building blocks, in harmony, to bring these life-altering medicines to patients.
Figure #1: As the CAGT pipeline grows, manufacturers keep facing challenges in bringing these life-changing therapies to patients.
Part 2: Building blocks for CAGT success
1. Patient discovery
Overview
Rare diseases are collectively not that rare; ~400 million people globally are affected. However, a single rare disease may affect as few as 10 people per year in the USA. Finding patients who may be eligible for a CAGT for a rare disease can be challenging, they are likely to be sparsely distributed across a country and may not even have confirmation of their diagnosis. On average a pediatric patient with a rare disease takes 6-8 years to have their diagnosis confirmed. Coupled to the known challenges of recruiting patients into clinical trials to demonstrate the value of innovative new medicines, discovering eligible patients is critical.
With over half of all CAGT trials focused on rare diseases, patients need to be matched to the most appropriate medicine. Therefore, manufacturers must develop ways to efficiently and effectively discover patients who may be eligible for their medicine, before their disease can progress further and patients are unable to benefit.
Solution / Case Study
Most infants in developed countries undergo newborn screening for a variety of different diseases, the breadth of analysis is usually limited by many factors which range from availability of tests to the amount of available sample. Newborn screening using next-generation sequencing could significantly expand the number of diseases which can be identified, however, this approach is still in its infancy.
Many of the rare diseases which can be addressed by CAGT are hereditary, connecting and leveraging family connections and genealogy to identify potential patients is a powerful approach for early patient identification. Biogen has successfully sponsored a registry for Spinal Muscular Atrophy (SMA) patients with sites in the US, UK and Italy, to collect data on patient and family history, to help track and further identify patients with SMA. This registry is also being used to track how patients are responding to Biogen’s SMA treatment, Spinraza.
2. Evidence generation
Overview
The clinical benefits of CAGT are evident and stories abound of the benefits to patients. Clinical evidence to demonstrate the benefit of CAGT to patients requires many elements to be orchestrated; treating a sufficiently meaningful number of patients, ensuring that the treatment effect can be compared to the current standard of care and that the durability of the clinical benefit is established. Three approaches are being leveraged which can help address these challenges; virtual clinical trials, synthetic trial arms and Real World Evidence.
Virtual clinical trials, a decentralized approach, leverages telemedicine, home health visits and digital devices to treat patients remotely, making it possible to engage with a more diverse patient population, accelerate recruiting, lowering the burden of trial participation and even improving quality and patient safety.
Synthetic trial arms enable the use of historical patient data as a comparison for a medicine, rather than using a parallel, placebo control group. Synthetic arms have multiple benefits; the data for the comparison may already exist, trials require fewer participants so can be completed more efficiently and they can be a more ethical approach. It may not be appropriate to give a placebo to a patient when you know that there may be a cure available for their life-limiting disease.
Medicines that confer many years of benefit are unlikely to be able to demonstrate this benefit in a classical clinical trial. Real World Evidence which supports the durability and magnitude of benefit can be anticipated to be required for most CAGT following launch. These so called ‘long term follow-up studies’ are also requirements for the FDA, EMA and European Commission, to monitor any delayed adverse events up to 15 years post trial.
The curative potential of CAGTs is implicitly difficult to demonstrate given the time-bound nature of any clinical study. Following effective treatment, patients move from being ‘patients’ to being ‘people’ and might either stop or decrease interactions with their treatment team. Thus, identifying ways to incentivize patients to follow up and opt into patient registries will be key to collecting the necessary data to address regulatory requirements, especially in times where multiple “one shot or close” therapies are racing towards the same indication.
Ultimately, manufacturers need to go beyond the current standard of care and consider what is appropriate for the future, provide a robust assessment of the benefit and plan out follow up studies and Real World Evidence strategies to demonstrate durability of their data and benefit.
Solution / Case Study
When properly designed, effective strategies for evidence generation resonate strongly with regulatory bodies and help alleviate some concerns around efficacy and safety. The German Healthcare Technology Assessment evaluation of Novartis Gene Therapies’ (formerly AveXis) SMA drug Zolgensma offers a great example. Zolgensma received a positive assessment, conditional on the continuous collection of Real World Evidence through robust patient registries. AveXis, whom Novartis acquired in 2019, set up their own patient registry (RESTORE) which is a prospective, multi center, multinational, non-interventional, long-term observational study of patients with SMA. The inclusion of trial participants into such registries, at a national or international level, will be key to ensure the necessary follow-up data is collected.
3. Manufacturing and logistics
Overview
The Cell and Gene Therapy manufacturing process is more complex than small molecules or biologics due to the requirement of viral or live biological material to be consistently produced and shipped at scale. This renders the process multipart and logistically complex, including potentially multiple quality control requirements. Additionally, commercial product scaling opportunities prove challenging and the lack of proper scaling often leads to difficulties for timely delivery due to slot capacity constraints and shipping logistics. This is particularly true for autologous products, which currently make up a significant portion of cell therapies in the market.
Additionally, there is a global dearth of cell and gene therapy manufacturing capacity; restricting the options which are available to developers and ultimately patients. As most processes are complex and require highly skilled personnel, it is important for manufacturers to begin the asset development with commercial scaling in mind. Innovative ways of leveraging centers of excellence with trained personnel familiar with the process can increase early success. Ultimately the process needs to be highly standardized to limit risks of batch to batch variability, scalable to anticipate capacity issues and strategically defined to include a robust supply chain for delivery.
Solution / Case Study
Autologous cancer cell therapies are prone to extended manufacturing times, due to the multitude of steps from apheresis to infusion, often 3-4 weeks elapse from therapy initiation to delivery. This timeline can result in some patients becoming too sick or dying while waiting for their treatment. For example, cancer patients eligible for CAR-T therapy, who usually have a remaining life expectancy of just a few months, may need to wait 31 – 80 days from the initial evaluation to administration due to various manufacturing and logistical hurdles (Figure 1).
Gilead has looked to address multiple challenges along the manufacturing process for their CAR-T therapy. Yescarta is only administered in certified centers where there is a dedicated and trained staff to assist the patient from evaluation to follow-up, reimbursement approval and manufacturing logistics. The manufacturing success rate is at 97%-99% with approximately a 17 days turnaround time in the US. Leveraging these learnings, Gilead is also scaling its manufacturing capabilities with the building of a plant in the Netherlands, allowing for a strong capabilities and shorter production times in the EU.
4. Regulatory approval
Overview
Lack of experience with the new modalities and uncertainty around long-term safety and efficacy are key concerns of regulators when assessing CAGTs. Manufacturers are required to provide extensive quality and safety data, yet agencies remain cautious in their decision-making process.
As more and more CAGTs successfully navigate the review process, new learnings and increasingly standard approaches are emerging. Since many CAGTs address serious conditions with high unmet need, they may be eligible for accelerated programs, which offer priority review, in addition regular touchpoints between manufacturers and regulators are important; providing timely advice on data requirements for approval.
Developing a precise, clearly defined plan based on deep understanding of regulatory requirements in close alignment with regulatory agencies is key to gaining accelerated approval indications and further ensuring capture of meaningful endpoints to secure launch.
Solution / Case Study
Spark Therapeutics’ Luxturna was approved by the FDA in 2017 to treat a rare form of blindness. It was the first directly administered gene therapy to be approved in the US targeting a disease caused by mutations in a specific gene. Instead of using standardized tests for their primary efficacy endpoints, Spark and the FDA jointly developed a new method, the so-called multi-luminance mobility test (MLMT), to simulate activities of everyday life and assess patients’ mobility. In addition to the Orphan Drug Designation, Luxturna had also received Priority Review and Breakthrough Therapy status, both of which allowed for frequent interaction with the regulators, but also expedited development and review of the therapy. The transparent dialogue between Spark and the FDA proved key in addressing uncertainties in the data early on and securing a positive decision.
5. Value and access
Overview
The well-established value frameworks for traditional therapies do not suffice for the evaluation of CAGTs. These focus on direct medical benefit, neglecting the broader value CAGTs offer to patients and society by preventing the development of disabling or life-threatening conditions. Yet, individual countries are beginning to establish specialized programs to overcome the regulatory hurdles for high cost rare disease drugs which address significant unmet need. NICE’s Highly Specialized Technology program is an example for such a scheme, under which gene therapies like Spark’s Luxturna have received a positive appraisal.
In addition to regulators, payers’ focus on short-term budgetary impact does not allow them to appreciate the long-term value offered by CAGTs, despite their significant medical value as one-off curative/disease-modifying therapies. Health insurers are also struggling with the high upfront cost of CAGTs due to their 3-5 year budgetary cycles and siloed social and healthcare budgets. To address payers’ concerns, manufacturers are entering novel financing and reimbursement agreements, which account for the long-term benefits and yet provide the payers with flexibility to share and spread the risks associated with CAGTs. These novel agreements include outcome-based models, where reimbursement is conditional on patient performance, as well as payment by installment.
Ultimately, it is the responsibility of manufacturers to develop a well-defined value proposition in early communication with payers to be able to discuss partnerships and payment models, while at the same time thinking proactively to remove patient access hurdles, leveraging patient support programs and solutions.
Solution / Case Study
Understanding the need to provide a solid base to allow payers to support their groundbreaking treatment, Bluebird Bio designed a novel agreement with German payers for its gene therapy Zynteglo, where reimbursement is split into five instalments. The first instalment is due once the patient received the treatment, while the remaining payments are spread over the following four consecutive years. However, these are only payable for those patients who remain transfusion-free, ultimately demonstrating confidence in their long-term efficacy and reinforcing Zynteglo’s price decision.
6. Advocacy and uptake
Overview
CAGTs often lack long-term data which can contribute to uncertainty about associated benefits and risks. Payers can be wary about reimbursing a treatment where the durability of outcomes may have some question marks. Physicians may have concerns about recommending and prescribing a therapy when there may be alternative options to consider. Patients and families become concerned with the ambiguity.
With these uncertainties and complexity, it is important to drive not only treatment awareness but disease awareness. Strong relationships with patient and family support programs that provide financial, logistical, and emotional support throughout the entirety of the process are vital. This will be increasingly important as products become available to be administered at a larger number of centers, establishing support lines in place for physicians, nurses, and case managers help navigate the complexities and increase uptake.
Ultimately manufacturers must focus on advocacy to enhance knowledge of therapy value, design fit-for purpose in-field Teams that support the stakeholders across the whole treatment paradigm and ensure close coordination with Centers of Excellence, which will be the first line treatment centers for many of the CAGT therapies.
Solution / Case Study
Novartis understood the complexity of this process and the need for stakeholder guidance and launched the Zolgensma OneGene Program to drive provider and patient advocacy, and financial assistance program. This program has a dedicated team that ensures the family has support from the point of prescription throughout the administration and follow-up period. Each family is assigned a patient resource manager that remains the point of contact for logistical and emotional support. There is a group of field reimbursement managers that navigate insurance requirements and manufacturing and case coordinators work closely with the doctors on reimbursement challenges. All work together in a highly interlinked way to ensure patients receive the support they need while receiving the lifesaving treatment.
Figure #2: Key Cell and Gene Therapy Building Blocks of Success
Part 3: Conclusion
CAGTs offer a new and exciting therapeutic paradigm, to which application of classical pharma commercialization approaches inevitably leads to suboptimal results.
To realize their full market potential, CAGT development cannot follow a traditional siloed model of sequential priorities (manufacturing, pricing, distribution etc.), as the success requirements are much more interlinked compared to existing therapies.
Due to the numerous dependencies, there is very little that can be properly done in isolation and the classical modular approach needs to be re-imagined. With the novelty of CAGTs and their challenges, standard solutions do not (yet) exist and manufacturers must come up with individualized, fit-for-purpose approaches.
The above distilled building blocks for success present a solid base for thinking through the key requirements for optimal, long-term commercialization and realizing the full potential of these phenomenal therapies.
"…cell-based and gene therapy technologies hold tremendous promise for addressing some of the most intractable diseases. But with their novelty, also come new uncertainties and some unique, theoretical risks" - Scott Gottlieb, M.D. – Former FDA Commissioner1
1Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies
Related solutions
Discover unrealized connections and manage your product across the entire lifecycle, from research and development, compliance to launch and commercialization.
Explore our end-to-end, full service clinical development capabilities including Therapeutics expertise, Development Planning, Phase I early clinical development, Phase IIb/III and Phase IV Trials, Regulatory Submission and Post-Launch Studies.





