Learning Management
Take Control of Clinical Trial Training
Give your sites the courses and credits they need to succeed with the Learning Management module of the Investigator Site Portal.
Give your sites the courses and credits they need to succeed with the Learning Management module of the Investigator Site Portal.



The Great Resignation has hit clinical research sites hard. With fewer and less experienced site staff available to run your trials, your training curriculum needs to be more effective than ever. But asking site staff to take redundant GCP and protocol training is counterproductive and frustrating.
Without a new approach to learning management across your clinical programs, training compliance will decline while protocol deviations climb, putting your trials at risk.

You may have tried to eliminate paper/email-based site-training with a general-purpose or corporate learning management system (LMS). But it doesn’t have the features you need to distribute courses and track compliance through the complexity of site roles and regulatory inspections.
What if you could have an enterprise-level LMS that was built specifically for training sites across all your protocols, therapeutic areas, and geographies - and gave you all the features you need for complete oversight plus high site satisfaction? Could your studies start-up faster and run more smoothly?
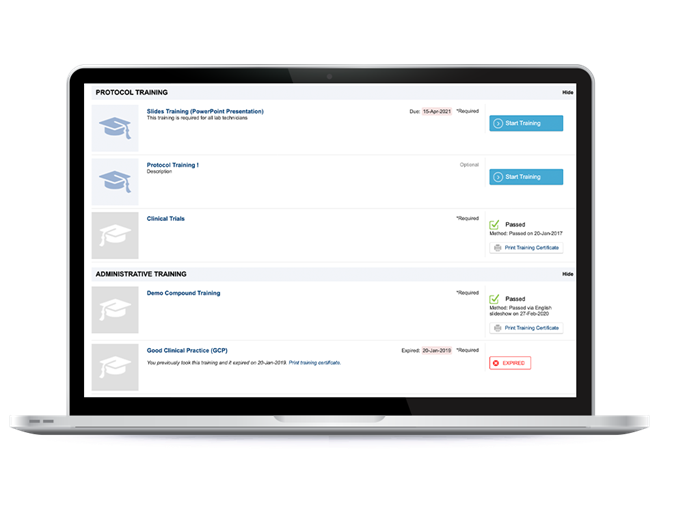
The LMS module of the IQVIA Investigator Site Portal gives you a centralized solution for distributing training courses to investigators and site staff that are studying your compounds around the world.
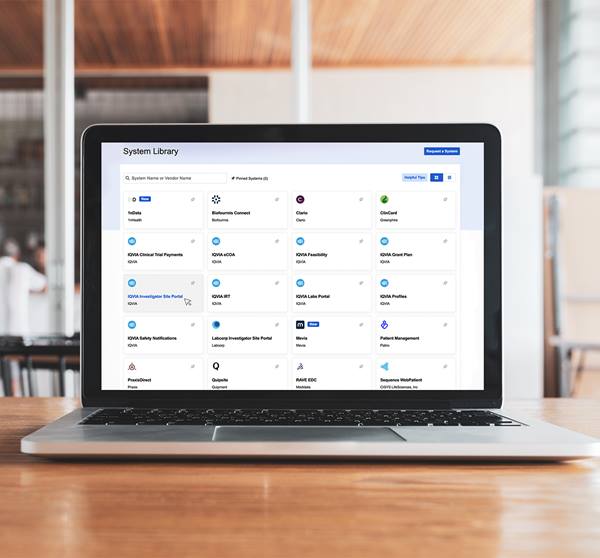


Increase transparency, improve communications and reduce administrative burden for all clinical trial stakeholders.
Communication, collaboration and transparency between sites and sponsors mean trials start up and close out faster.
Communicate SAEs, SUSARs, and other significant events to sites around the world with the Safety Notifications module of the IQVIA Investigator Site Portal.